Summary
Here we describe an in vitro co-culture system that can differentiate hematopoietic progenitor populations to all major hematopoietic lineages at clonal level. We present both a sensitive single-cell switch-culture system as well as a less laborious alternative barcoding protocol more convenient for larger cell numbers. Importantly, generation of all lineages from single long-term hematopoietic stem cells are described, following 21 days of culture. This protocol represents an efficient tool for validation experiments for single-cell genomics data.
For complete details on the use and execution of this protocol, please refer to Safi et al. (2022).1
Subject areas: Bioinformatics, Cell Biology, Cell culture, Cell isolation, Flow Cytometry/Mass Cytometry, Genomics, Molecular Biology, Stem Cells
Graphical abstract

Highlights
-
•
Protocol for clonal in vitro multilineage differentiation of hematopoietic progenitors
-
•
Single-cell culture for sensitive analysis of purified long-term hematopoietic stem cells
-
•
Flow cytometry readout of B cells, myeloid cells, erythrocytes, and megakaryocytes
-
•
Barcoding protocol and novel analysis pipeline (Bartide) for high-throughput cultures
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Here we describe an in vitro co-culture system that can differentiate hematopoietic progenitor populations to all major hematopoietic lineages at clonal level. We present both a sensitive single-cell switch-culture system as well as a less laborious alternative barcoding protocol more convenient for larger cell numbers. Importantly, generation of all lineages from single long-term hematopoietic stem cells are described, following 21 days of culture. This protocol represents an efficient tool for validation experiments for single-cell genomics data.
Before you begin
To enable evaluation of the clonal capacity of hematopoietic stem and progenitor cells into various lineages, this protocol describes a culture system designed to allow for parallel differentiation into B, Myeloid, or Erythroid cells at single-cell resolution. All the steps described here are carried out in a laminar flow class II biological hood, using proper aseptic techniques to avoid contamination.
The main steps consist of, 1) Preparation of OP9 cells, 2) Isolation of Bone Marrow (BM) from adult mice, and cell sorting of distinct test populations, 3) Differentiation cultures, and 4) Readout of cells by flow cytometry or cell sorting. The alternative protocol also includes transduction with barcoding-carrying viruses as well as sequencing and bioinformatic data analysis. While allowing for the analysis of higher cell numbers, the barcoding protocol is unstable when assaying purified long-term hematopoietic stem cells (LT-HSCs), introducing technical variation. This is likely due to viral transduction being inefficient in HSCs and/or negatively impacting stem cell function. Thus, we recommend the switch culture for assaying purified HSC populations, while the barcoding protocol is efficient and robust for the analysis of downstream hematopoietic progenitors.
Institutional permissions
In this protocol we have used 8–10-week-old, congenic mice (B6SJL (CD45.1) or C57BL/6 × B6SJL (CD45.1/CD45.2)). All experiments were approved by the Lund district court. Mice were kept in ventilated racks and given autoclaved food and water in the barrier facility at the Biomedical Center at Lund University.
Prepare OP9 stroma cells - One week before differentiation experiment
Timing: 1 h
-
1.
Sterilize the tissue culture hood and microscope using standard methods.
-
2.
Prepare the complete OptiMEM medium for cell culture, according to table “Cell culture medium” below.
-
3.OP9 murine stromal cells were stored frozen in −150°C, in freezing medium containing 90% FBS and 10% DMSO. Thaw cells according to the steps bellow:
-
a.Quickly thaw cells in 37°C water bath and transfer to a 15 mL tube.
-
b.Dropwise add 10 mL FBS to the cells, and spin down in 350 g for 5 min at 4°C.
-
c.Re-suspend the pellet in 1 mL fresh complete OptiMEM and count the cells to calculate recovery after freezing/thawing.Note: Here cells are counted using an Automated Cell Counter. Mix 10 μL of sample with 10 μL of trypan blue, pipette into a disposable Countess™ chamber slide, insert to machine and press count.
-
d.Seed 100,000 cells per T-75 culture flask, in 15 mL complete OptiMem.
-
e.Incubate cells at 37°C and 5% CO2, and passage at 80% confluence (after approximately 2 days).Note: It is important to allow the OP9 cells to recover after thawing. We recommend 2–3 passages prior to performing experiments. Moreover, OP9 cells should not be used for more than 1 month after thawing (approximately 15–17 passages). Finally make sure that the OP9 cells maintain the morphology according to Figure 1A. Confluence >80% will result in differentiation and loss of function (Figure 1B).
-
a.
Figure 1.
Morphology of OP9 stroma cells
(A) OP9 cells at <80% confluence.
(B) OP9 cells over-confluent >80% (do not use), scale-bare represent 200 μm.
Preparation before sorting cells- Day 0
Timing: 0.5 day
-
4.Seeding OP9 cells.
-
a.Sterilize the tissue culture hood and microscope (see step 1).
-
b.Split OP9 cells at 80% confluency.
-
i.Remove the medium from the flask, rinse the adhered cells with 10 mL PBS, gently swirl the flask to remove the serum.
-
ii.Remove the PBS and add 1 mL 0.05% Trypsin to a 75 cm2 flask of cells, gently swirl the flask to cover the cells evenly with Trypsin and incubate for 3 min at 37°C.
-
iii.Tap the flask to loosen the cells and check in the microscope that cells have detached. Add 10 mL complete OptiMEM and transfer cells to a 50 mL falcon tube. Rinse the flask with another 10 mL medium and transfer to the same 50 mL Falcon tube.Note: to deactivate the Trypsin, it is important to add the medium containing serum.
-
iv.Centrifuge the cells in 350 g for 5 min, remove supernatant, add 1 mL complete OptiMEM and count the cells.
-
i.
-
c.Seed 2000 OP9 cells per well in 100 μL complete OptiMEM supplemented with cytokines (50 ng/mL SCF and 50 ng/mL Flt3L) in flat bottom 96-well culture plates with low evaporation lid. To maintain humidity one row surrounding cell-containing wells are filled with autoclaved water. Incubate at 37°C and 5% CO2.Note: Complete OptiMEM medium can store at 4°C for up to 1 month.For the “Alternative protocol” (barcoding), step c is slightly modified, and an additional step (d) has been added as well (see below):
-
d.Seed 6000 OP9 cells per well in 150 μL complete OptiMEM without cytokines in flat bottom 48-well culture plates (called HSC plate) with low evaporation lid.Note: OP9 cell number should be adjusted based on the plate's cm2 area.
-
e.Prepare cytokine mix appropriate for a final volume of 300 μL, in 150 μL complete OptiMEM per well (store at 4°C until use) according to the table “Alternative 2- cytokine” in the “materials and equipment”.Note: Cytokine can store at 4°C for up to 1 week.
-
a.
-
5.Cell sorting preparation:
-
a.Prepare FACS-buffer as shown in table “FACS buffer” in “materials and equipment”.
-
b.Prepare staining cocktails for FACS (store at 4°C), including single cell compensation controls and Fluorescence Minus One (FMO) controls as shown in table “Antibody cocktail for cell sorting” in “materials and equipment”.
-
a.
Note: The staining cocktail presented here is for sorting of Hematopoietic stem cells (HSCs) but can be substituted with other colors for your population of interest. We use ultra Comp eBeads for compensation controls in this study.
-
6.
Autoclave dissection tools (pestle and mortar, Eppendorf tubes).
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rat anti –mouse Sca1-PE-CY7 (clone D7) | BioLegend | Cat#108114, RRID: AB_493596 |
| Rat anti –mouse cKit (cd117)-APC (clone 2B8) | BioLegend | Cat#105812, RRID: AB_313221 |
| Rat anti –mouse CD135 (FLT3)-PE (clone A2F10) | BioLegend | Cat#135305, RRID: AB_1877218 |
| Rat anti –mouse CD135 (FLT3)- BV-421 (cloneA2F10) | BioLegend | Cat#135315, RRID: AB_2571919 |
| Rat anti –mouse CD34- FITC (cloneRAM34) | BD Bioscience | Cat#560238, RRID: AB_1645242 |
| Rat anti –mouse CD16/32 (FC block) | BD Bioscience | Cat#553142, RRID: AB_394657 |
| Rat anti –mouse CD41- PE (cloneMWReg30) | BioLegend | Cat#133905, RRID: AB_2265179 |
| Rat anti –mouse CD11b- APC (cloneM1/70) | BioLegend | Cat#101211, RRID: AB_312794 |
| Rat anti –mouse CD11b- PE-CY5 (cloneM1/70) | BioLegend | Cat#101209, RRID: AB_312792 |
| Rat anti –mouse LY-6G/LY-6C (GR1) BV-605 (cloneRB6-8C5) | BioLegend | Cat#108439, RRID: AB_ 2562333 |
| Rat anti –mouse LY-6G/LY-6C (GR1) APC-CY7 (cloneRB6-8C5) | BioLegend | Cat#108423, RRID: AB_ 2137486 |
| Rat anti –mouse F4/80 APC-CY7 (cloneBM8) | BioLegend | Cat#108423, RRID: AB_ 2137486 |
| Anti –mouse/human CD45R/B220 PE-CY5 (cloneRA3-6B2) | BioLegend | Cat#103209, RRID: AB_ 312994 |
| Rat anti –mouse CD3 PE-CY5 (clone145-2C11) | BioLegend | Cat#100273, RRID: AB_ 2894410 |
| Rat anti –mouse TER119 PE-CY5 (cloneTER119) | BioLegend | Cat#116209, RRID: AB_ 313710 |
| Rat anti –mouse TER119 PE-CY7 (cloneTER119) | BioLegend | Cat#116221, RRID: AB_ 2137789 |
| Rat anti –mouse NK1.1PE-CY5 (clonePK139) | BioLegend | Cat#108716, RRID: AB_ 493590 |
| Rat anti –mouse CD45 Alexa Fluor 700(clone30-F11) | eBioscience | Cat#56-0451-82, RRID: AB_ 891454 |
| Rat anti –mouse CD19- PE-CY7 (clone1D3) | eBioscience | Cat#25-0193-81, RRID: AB_657664 |
| Rat anti –mouse CD61-BV421 (clone 2C9.G2) | BD Bioscience | Cat#566227, RRID: AB_2739615 |
| Bacterial and virus strains | ||
| LV-GFP-Lib (Lentiviral) | Laboratory of Dr, Joan Yuan | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Mouse recombinants SCF | PEPROTHECH | Cat# 250-03 |
| Mouse recombinants FLT3-ligand | PEPROTHECH | Cat# 250-31L |
| Mouse recombinants TPO | PEPROTHECH | Cat# 315-14 |
| Mouse recombinants IL-7 | PEPROTHECH | Cat# 217-17 |
| Holo-Transferrin human | SIGMA | Cat# T0665-50MG |
| EPO | Laboratory of MD/Dr. Johan Flygare | N/A |
| 7AAD | BD Bioscience | Cat#559925 RRID: AB_2869266 |
| UltraComp eBeads | Invitrogen | Cat# 01-2222-42 |
| Qualified FBS | Gibco | Cat#26140079 |
| DPBS, Ca2+/Mg2+ free | Gibco | Cat#14190169 |
| Pencillin/Streptomycin | Gibco | Cat#15140122 |
| Opti-MEM with GlutaMAX | Gibco | Cat# 51985-034 |
| 2-mercaptoethanol (50 mM) | Gibco | Cat#31350-010 |
| Trypan Blue (0.4%) | Thermo Fishet | Cat#15250061; CAS:72-57-1 |
| 0.05% Trypsine-EDTA (1×) | Gibco | Cat#2530054 |
| EDTA 0.5 M | Gibco | Cat#15090046 |
| Nuclease free water | Ambion | Cat# AM9937 |
| AMPure XP | BECKMAN COULTER | Cat# A63881 |
| Proteinase K | PCR grade | Cat#Eo0491 |
| Q5® High-Fidelity DNA Polymerase sets (2 U/μL) | New England BioLabs | Cat# M0491S |
| Q5® Reaction Buffer (5×) | New England BioLabs | Cat# B9027S |
| 10 mM dNTP mix | Thermo Fisher | Cat#R0192 |
| May-GRünwad | SIGMA | Cat# MG500-500ML |
| Gimsa | Merck | Cat# 1.09204.0500 |
| Critical commercial assays | ||
| ANTI-APC Micro Bead kit-mouse | Miltenyi Biotec | Cat# 130-090-855 |
| QuadroMACS separator | Miltenyi Biotec | Cat# 130-090-979 |
| MACS LS Columns | Miltenyi Biotec | Cat#130-042-401 |
| MiSeq- reagent kit- v3- 150 cycle | Illumine | Cat #MS-102-3001 |
| Bioanalyzer HS DNA Analysis Kit | Agilent | Cat#5067-4626 |
| Bioanalyzer HS DNA Analysis regent | Agilent | Cat#5067-4627 |
| Deposited data | ||
| Raw Mi-Seq for barcoding | This paper | https://osf.io/r5h38/ |
| Experimental models: Cell lines | ||
| Mouse: OP9 | Laboratory of Profs, David Bryder | N/A |
| Experimental models: Organisms/strains | ||
| Mouse: B6JL (8–10 week, Female/Male) | In-house breeding | N/A |
| Mouse: C57BL/6 × B6SJL (8–10 week, Female/Male) | In-house breeding | N/A |
| Oligonucleotides | ||
| BCErv primer: 5′GTCTCGTGGGCTCGGAGATGTGT ATAAGAAACAGGACGGCATACGAGCTCTTCC-3′ |
Laboratory of Dr, Joan Yuan | N/A |
| LibID primer: 5′TCGTCGGCAGCGTCAGATGTGTATA AGAGACAGTACACGACGCTCTTCCGATC-3′ |
Laboratory of Dr, Joan Yuan | N/A |
| Software and algorithms | ||
| FlowJo –V10.1 | BD Bioscience | https://www.flowjo.com/ |
| Prism | Graph Pad | https://www.graphpad.com/scientific-software/prism/ |
| PYTHON (V 3.9) | https://www.python.org/downloads/ | |
| Microscope | Olympus, BX51 | N/A |
| Other | ||
| Cell culture flask T-75 cm2 | Corning | Cat#430641U |
| Tissue culture 96 well plate | TPP | Cat#92096 |
| Tissue culture 48 well plate | TPP | Cat#92048 |
| Tissue culture 6 well plate | TPP | Cat#92006 |
| V- bottom 96 well plates | Thermo Scientific | Cat#249944 |
| 10-cm tissue culture dish | Falcon | Cat#353003 |
| Shandon Cytospin 3 | Block Scientific | N/A |
| Cell strainers, 40 μm | Corning | Cat#431750 |
| Cup filter, 50 μm | BD-BD Biosciences | Cat#340630 |
| Autoclaved 1.5 mL Eppendorf tubes | Fisher Scientific | Cat#05-405-129 |
| FACS tubes | Corning, Falcon | Cat#352058 |
| Disposable PES Filter Units (500 mL) | Fisherbrand | Cat#15913307 |
| 8- Multichannel pipette | Thermo Scientific | N/A |
Materials and equipment
| Complete OptiMEM | Final concentration | Amount |
|---|---|---|
| OptiMEM with Glutamax | No | 444.5 mL |
| FBS | 10% | 50 mL |
| 2-mercaptoethanol (50 mM) | 0.1% | 500 μL |
| Pen-strep | 1% | 5 mL |
| Total | N/A | 500 mL |
Filter and store at 4°C for up to 1 month.
| Complete SFEM | Final concentration | Amount |
|---|---|---|
| SFEM | No | 345 mL |
| FBS | 30% | 150 mL |
| Pen-strep | 1% | 5 mL |
| Total | N/A | 500 mL |
Filter and make aliquots, store at −20°C and thaw in time of use.
| FACS buffer | Final concentration | Amount |
|---|---|---|
| PBS | No | 488 mL |
| FBS | 2% | 10 mL |
| EDTA (0.5 M) | 2 mM | 2 mL |
| Total | N/A | 500 mL |
Filter and store at 4°C for up to 1 month.
| Antibody-cell sorting | Fluorochrome | Dilution |
|---|---|---|
| Lineage antibodies: CD3, GR1, TER119, B220, CD11b, NK1.1 |
PE-CY5 | 1:400 each |
| C-KIT | APC | Added in C-kit enrichment step |
| SCA1 | PE-CY7 | 1:200 |
| CD48 | APC-CY7 | 1:100 |
| CD150 | BV-605 | 1:200 |
| Flt3 | BV-421 | 1:100 |
Alternative 1- Antibody cocktail for lineage read out
| Antibody–ER condition | Fluorochrome | Dilution |
|---|---|---|
| CD45 | Alexa Fluor 700 | 1:400 |
| GR1 | BV-605 | 1:200 |
| MAC1 | APC | 1:300 |
| F4-80 | APC-CY7 | 1:100 |
| TER119 | PE-CY7 | 1:200 |
| Antibody–B condition | Fluorochrome | Dilution |
| CD45 | Alexa Fluor 700 | 1:400 |
| GR1 | BV-605 | 1:200 |
| MAC1 | APC | 1:300 |
| F4-80 | APC-CY7 | 1:100 |
| CD19 | PE-CY7 | 1:400 |
Alternative 1- Cytokines
| Cytokine–B condition | Final concentration |
|---|---|
| SCF | 50 ng/mL |
| Flt3L | 50 ng/mL |
| IL7 | 20 ng/mL |
Store at 4°C for up to 1week.
| Cytokine–Erythroid condition | Final concentration |
|---|---|
| SCF | 50 ng/mL |
| Flt3L | 50 ng/mL |
| EPO | 3 U/mL |
| Holo-transferrin (100 mg/mL) | 0.3 mg/mL |
Store at 4°C for up to 1week, except for Holo-transferrin, which is added just before use.
Alternative 2- Cytokines
| Cytokine | Final concentration |
|---|---|
| SCF | 50 ng/mL |
| Flt3L | 50 ng/mL |
| IL7 | 20 ng/mL |
| EPO | 3 U/mL |
| Holo-transferrin (100 mg/mL) | 0.3 mg/mL |
Store at 4°C for up to 1week, except for Holo-transferrin, which is added just before use.
Alternative 2- Antibody cocktail for lineage read out
| Antibody | Fluorochrome | Dilution |
|---|---|---|
| CD45 | Alexa Fluor 700 | 1:400 |
| GR1 | BV-605 | 1:200 |
| MAC1 | APC | 1:300 |
| F4-80 | APC-CY7 | 1:100 |
| TER119 | PE-CY7 | 1:200 |
| CD41 | PE | 1:400 |
| CD61 | Bv-421 | 1:200 |
FACS cell sorter and analyzer
| Instrument | Laser wavelength | Laser power | PMTs and filter configurations |
|---|---|---|---|
| BD FACS AriaIII | Violet 405 nm | 30 mW | A:780/60;B:710/50;C:665/30;D:610/20;E:510/50;F:450/40 |
| Blue 488 nm | 20 mW | A:680/13;B:525/50;C:488/10 | |
| Yellow-Green 561 nm | 50 mW | A:780/60;B:710/50;C:670/14;D:610/20;E:582/15 | |
| Red 640 nm | 17 mW | A:780/60;B:730/45;C:660/20 | |
| BD LSR Fortessa X20 | UV 355 nm | 15 mW | A:525/50;B:379/20 |
| Violet 405 nm | 50 mW | A:780/60;B:710/50;C:670/30;D:610/20;E:525/50;F:450/50 | |
| Blue 488 nm | 60 mW | A:710/50;B:530/30;C:488/10 | |
| Yellow-Green 561 nm | 50 mW | A:780/60;B:710/50;C:670/30;D:610/20;E:586/15 | |
| Red 640 nm | 40 mW | A:780/60;B:730/45;C:670/30 |
Alternative 2- Lysis buffer
| Reagent | Final concentration |
|---|---|
| Tris-HCL (PH 8.5) | 0.1 M |
| EDTA (PH 8) | 0.5 mM |
| NaCl | 0.2 M |
| SDS | 0.2% |
| Proteinase K | 0.1 mg/mL (Add immediately before use) |
Alternative 2- PCR#1- Reaction master mix
| Reagent | Amount |
|---|---|
| DNA template | 14 μL (1.43 ng/μL) |
| DNA Q5 HF polymerase | 0.5 μL (2 U/μL) |
| Primer1 | 2.5 μL (1 μM) |
| Primer2 | 2.5 μL (1 μM) |
| Q5 reaction buffer | 10 μL (5×) |
| dNTP mix | 1 μL (10 mM) |
| ddH2O | 19.5 μL |
| Total | 50 μL |
Alternative2- PCR#1- Cycling condition
| Steps | Temperature | Time | Cycles |
|---|---|---|---|
| Initial Denaturation | 98°C | 30 s | 1 |
| Denaturation | 98°C | 10 s | 30 cycles |
| Annealing | 72°C | 1 min | |
| Extension | 72°C | 2 min | 1 |
| Hold | 4°C | Forever | |
Alternative2- PCR#2- Reaction master mix
| Reagent | Amount |
|---|---|
| DNA template | 14 μL (0.143 ng/μL) |
| DNA Q5 HF polymerase | 0.25 μL (2 U/μL) |
| i7+i5 primers (IDT for Illumina Unique Dual Indexes) | 5 μL |
| Q5 reaction Buffer | 5 μL (5×) |
| dNTP mix | 0.5 μL (10 mM) |
| ddH2O | 0.25 μL |
| Total | 25 μL |
Alternative2- PCR#2- Cycling condition
| Steps | Temperature | Time | Cycles |
|---|---|---|---|
| Initial Denaturation | 72°C | 3 min | 1 |
| Initial Denaturation | 98°C | 3 min | 1 |
| Denaturation | 98°C | 45 s | 8 cycles |
| Annealing | 62°C | 30 s | |
| Extension | 72°C | 1 min | |
| Final extension | 72°C | 2 min | 1 |
| Hold | 4°C | Forever | |
Step-by-step method details
For the standard cell differentiation protocol, follow steps 1-20. For the alternative barcoding protocol, please follow steps 1-3 and then skip to step 21.
Cell sorting- Day 1
Timing: 1 day
In this step your population of interest is sorted as single cells in 96-well plates.
-
1.Harvest BM.
-
a.In the morning, euthanize mouse according to your ethical permit.
-
b.Dissect BM containing bone such as pelvis, femur, tibia-fibula, radius-ulna, humerus, spine (vertebra), scapula, and ribs from mice and collect these in a 50 mL tube containing cold (4°C) FACS-buffer.Note: In our experience tibia, femur and pelvis contain the highest number of BM cells.
-
c.Crush bones in ice-cold FACS-buffer with a mortar and pestle and make single-cell suspensions by gently pipetting up and down using a 1 mL pipette tips.
-
d.Filter through a 40 μm cell strainer. Count the cells and centrifuge at 350 × g for 5 min at 4°C.
-
e.Remove the supernatant and resuspend cells in FACS-buffer, 1 mL/250 × 106 cells.
-
f.Set aside around (10k) cells for “FMO APC” and “Unstained control”. The remaining cells will be used for C-Kit-enrichment.Note: This step is important to enrich for LSK population and reduces sorting time. Lineage depletion also can replace C-kit enrichment in this step.
-
a.
-
2.C-Kit-enrichment.
-
a.Add 2 μL c-Kit-APC-antibody per 1 mL cell suspension.
-
b.Incubate samples at 4°C in darkness and gentle shaking for 30 min.
-
c.Wash cells by filling up the tube with FACS-buffer and spin 350 g for 8 min.
-
d.Re-suspend the cell pellet in 500 μL FACS-buffer with 25 μL anti-APC beads/250 × 106 cells.
-
e.Incubate dark at 4°C for 30 min. Shaking is not required.
-
f.Wash cells by filling up the tube with FACS-buffer and spin 350 g for 8 min.
-
g.Re-suspend cells in 500 μL FACS buffer.
-
h.For C-Kit-enrichment insert MACS column into the MACS separator magnet and put a 50 μm cup filter on top of each column.
-
i.Equilibrate the columns by running 3 mL FACS buffer through the column and discard the liquid flow through.
-
j.Place labeled collection-tubes underneath each column and pipette the 500 μL sample through the filter.
-
k.Rinse the sample-tube with 500 μL of buffer and add to column.
-
l.Add 3 mL of buffer to the column to rinse off the unbound cells, wait until the buffer run through then repeat this step two more times.
-
m.Separate the column from the separator magnet and place it on top of a new collection-tube.
-
n.Add 5 mL buffer to the column and flush out the cells by firmly pushing the supplied plunger.
-
a.
-
3.Cell staining.
-
a.Count the cells using an Automated Cell Counter.
-
b.Set aside around (200k) cells for the remaining “FMOs”.
-
c.Spin the cell suspensions at 4°C 350 g for 8 min; remove the supernatant.
-
d.Resuspend the cell pellet in FACS buffer containing FC-block (1:100) at the volume of 100 μL/107 c-Kit enriched cells and incubate for 5 min in 4°C.
-
e.Add antibody staining cocktail according to the table in the “materials and equipment” section.
-
f.Stain the sample and FMO controls and incubate dark for 45 min at 4°C and gentle shaking.
-
g.Wash cells in FACS buffer, spin at 350 g for 8 min at 4°C, remove supernatant.
-
h.Re-suspend pellet in FACS-buffer containing 7AAD (1:200), and re-suspend FMO PECy5 and unstained-control in FACS-buffer without 7AAD.
-
a.
Note: We sorted on an AriaIII instrument, according to configuration designed in “FACS cell sorter and analyser” in “materials and equipment” section.
-
4.Cell sorting.
-
a.Sort single cells (LT-HSCs) into 96-well plate containing the OP9-layer according to the gating strategy in (Figures 2A and 2B).Optional: Activate index sorting in the FACS sorting software to save the immunophenotypic profile for each single cell.Note: In this study we used 70 μm nozzle in AriaIII with the sort of precision mode 4-way purity (0-32-0). The sorting purity is recommended to be greater than 95% of viable cells (Figure 2B).
-
b.After sorting, wipe the surface of the plate with 70% ethanol and keep it in a closed container together with a petri culture dish containing autoclaved water.
-
c.Store in incubator at 37°C and 5% CO2 for 4 days.
-
a.
Figure 2.
FACS cell sorting gating strategy for hematopoietic stem and progenitor populations
(A) Representative FACS gating strategy for LSK, LTHSCs and LMPP.
(B and C) Purity check and FMOs have been shown in (B) for LTHSCs, LMPPs and (C) for LSK.
Preparation for switch culture- Day 3
Timing: 2–3 h
In this step the growing single-cell cultures will be split equally to Erythroid or B cells conditions according to the experimental design (Figure 3A). The 96 well plates used in this step should be with low evaporation lid.
-
5.
For B-cell differentiation culture, 2000 OP9 cells are seeded into a new flat bottom 96 well culture plate containing 50 μL complete OptiMEM with cytokine.
Note: The cytokine concentration should be appropriate for final volume of 200 μL according to the table “Alternative 1-cytokines B conditions”.
-
6.
Fill the wells surrounding the seeded cells with autoclaved water and incubate at 37°C and 5% CO2 preferably overnight or at least 6 h before sorting.
-
7.
For Erythroid (ER) differentiation culture, prepare a new flat bottom 96-well plate with 50 μL complete SFEM with cytokines.
Note: The cytokine concentration should be appropriate for a final volume of 200 μL according to the table “Alternative 1- cytokines Erytrhoid condition”.
-
8.
Fill the wells surrounding the SFEM wells with autoclaved water, keep them in 4°C overnight in a sterile container.
Note: This step can be prepared at day 4 as well.
Figure 3.
Methodology and FACS read out for the switch culture protocol
(A) Experimental strategy for clonal switch-culture.
(B and C) Representative FACS gating strategy of (B) LTHSCs-derived Myeloid (My), Macrophage (MΦ) and B clones from B condition at day 21 co-cultures, and (C) Myeloid (My), Macrophage (MΦ) and Erythoid (ER) clones for ER culture system at day 21.
(D) Lineage distribution of clones derived from single cells from the indicated sub-populations (LT-HSCs and LMPP). Error bars indicate standard deviation.
(E and F) Representative May-Gruwald-Giemsa staining of cytospin slides from the cells of readout in (E) Erythroid or (F) B condition at day 21. Scale-bare represents 20 μm.
Switch culture- Day 4
Timing: 0.5 day
-
9.
Sterilize the tissue culture hood and microscope according to step 1.
-
10.
Check the plate with sorted cells after 4 days under the microscope to verify the growth of clones.
Note: At this stage the earliest colonies from certain progenitors such as LMPP may be visible; however, colonies from LT-HSCs will appear later. As a positive control seed a well with 10 cells while sorting.
-
11.
Place the plate with sorted cells as well as the plates prepared in steps 1 and 3 in the hood.
-
12.
Homogenize the cells in each well (100 μL) by pipetting with a Multichannel pipette.
-
13.
Divide 50 μL into B cell differentiation culture and 50 μL in ER cell differentiation culture (Figure 3A). Make sure to plate the cells in the same plate-position as in the (index) sorted plate.
-
14.
Rinse each well two times with 50 μL complete SFEM medium or OptiMEM medium without cytokines to recover all the cells.
-
15.
Divide the cells equally to each differentiation condition (the total volume in each condition should be 200 μL/well).
-
16.
Check the sorted plate under the microscope to ensure that no cells remain in the wells.
Note: If cells remain, rinse again with complete SFEM or OptiMEM medium and add to the respective well of each condition. Adjust cytokine concentration accordingly.
-
17.
Keep plates in a sterile container including a petri dish with autoclaved water in 37°C and 5% CO2 incubator until readout.
-
18.Change half the medium every 7-day.
-
a.Verify cell growth by microscope analysis.
-
b.Centrifuge the plate for 5 min in 350 g at 4°C.
-
c.Remove 100 μL medium from top carefully without interfering with the growing clone.
-
d.Add 100 μL fresh medium with cytokine concentration appropriate for 200 μL and incubate in 37°C and 5% CO2.
-
a.
Note: If the cells are confluent they should be moved to a larger-sized plate. In this case, homogenize the cells in a 96-well plate by pipetting the cells and transfer (200 μL/well) into the newly prepared 48-well plates, for B condition (containing OP9 cells) or erythroid condition, as previously explained. Rinse the 96-well plates two times with 100 μL medium supplemented with appropriate cytokine concentrations for a total of 400 μL, and transfer to the 48-well plate (in total 400 μL/well).
Read out-Day 21
Timing: 1 day
The kinetics of progenitors and lineage differentiation varies. It is important to optimize the time point for read out for the test population beforehand at bulk or single cell level.
Note: In our experience, the kinetics of bulk cells is slightly faster compared to single cells; therefore we recommend setting up the experiment at single cell level.
-
19.FACS analysis.
-
a.Homogenize the cells by pipetting up and down (using 8- Multichannel pipette), transfer cells to V- bottom 96 well plates.
-
b.Rinse each well once more with 100 μL of FACS buffer and add to the respective well in the V- bottom 96 well plates.
-
c.Centrifuge plates for 5 min in 350 g and discard supernatant without disturbing the cell pellet.
-
d.Re-suspend the pellets in 50 μL of FACS buffer containing FC-block (1:100) and incubate for 5 min at 4°C.
-
e.For Erythroid or B cell differentiation culture, respectively, stain cells according to the table “Alternative 1- antibody cocktail” and incubate for 45 min, in dark, 4°C on slow shaker.
-
f.Rinse stained cells with FACS buffer, centrifuge at 350 g for 5 min at 4°C.
-
g.Remove supernatant and re-suspended cells in 200 μL FACS buffer containing 7AAD (1:200).
-
h.Set up staining compensation matrix using single stained controls (beads) on LSR-Fortessa (BD).
- i.
-
j.Run samples and FMOs using plate or tube reader.Note: If a plate reader is used; the “maximum wash” setting should be applied between each sample according to the specific FACS instrument’s settings.
-
k.Following FACS, lineage output is analyzed in FlowJo for the presence of Erythroid cells (CD45-, F4-80-, Mac1-, Gr1-, TER119+), B cells (CD45+, CD19+, Mac1-) and myeloid cells (either CD45+, Mac1+, Gr1+, or CD45+, Mac1+, F-480+).Note: We set the cut-off to ensure that we accurately capture the population. For clones to be considered positive, the scatter profile must contain at least 50-gated events. Each lineage clone's population should be distinct in back gating, and more than 20 events must be recorded within the scatter profile for the analysis to score as positive.
-
a.
Verify cell type
Timing: 0.5 day
As an alternative the morphology of cells could be confirmed by May-Grünwald/Giemsa-staining (Figures 3E and 3F).
-
20.Cytospin and May-Grünwald/Giemsa-staining in brief.
-
a.Cells were Cytospinned using Shandon Cytospin 3, at 550 r.p.m with low acceleration for 3 min.Note: When spinning less than 1000 cells, pre-wet the filter with 50 μL PBS, spin at 550 r.p.m with low acceleration for 3 min, and load 100 μL of cell suspension.
-
b.Stain air-dried slides in May-Grünwald solution for 5 min.
-
c.After a brief wash with water, stain the slides in Giemsa solution for 10 min.
-
d.Wash the slide with dH2O and air dry before microscopic analysis.Note: In our hand, it is critical to dilute Giemsa with dH2O or miliQ water (1:10) and cover cuvette with a lid to reduce evaporation. Otherwise, a higher concentration of Giemsa will confound results.
-
a.
Alternative protocol 2 (barcoding protocol)
In this protocol, we have optimized the differentiation culture system and included barcoding, allowing for higher throughput and bulk culture. This protocol also allows for the differentiation and growth of all lineages without a switch in culture conditions (Figure 4).
Figure 4.
Summary of the barcoding protocol’s experimental workflow
Cellular barcoding is emerging as a powerful tool for addressing individual cell fates on a large scale by tagging individual cells of interest with unique heritable identifiers or barcodes. The generation of lentiviral barcode libraries, the significance of complexity and size of libraries, as well as analytical challenges, has all been carefully reviewed before.2,3,4 Prior planning is necessary to ensure an appropriate experimental design. The barcoding protocol used here adopts technology from Kristiansen et al.5 to track the multilineage differentiation of LSK cells in-vitro. However, this protocol is also useful for assaying committed progenitors as exemplified by the LMPP data in Figure 6B.
Figure 6.
Representative FACS sorting strategy at read out of barcoding cultures
(A and B) (A) Sort purity check and Representative May-Gruwald-Giemsa staining of cytospine slides for Myeloid (My), Macrophage (MΦ), B, Megakaryocyte (MK) and Erythroid (ER) form lineage output of LSK cells after 21 day or (B) LMPP cells after 8 day or 16 day of co-culture, Scale-bare represent 5 μm.
Preparation
The preparation step is performed as described above. Only steps 4c and 4d are different from switch culture and marked as Alternative protocol 2.
Please perform steps 1-3 above before continuing with step 21.
Cell sorting and transduction
Timing: 1 day
-
21.
Cell sorting.
We used a BDAria III instrument (configuration listed in “materials and equipment” section).-
a.Sort at least 2500 LSK (Lin-, Sca1+, C-kit+) or LMPP (LSK, CD34+, Flt3+) cells according to the gating strategy in Figure 2A, into prepared collection tubes containing 150 μL cold complete OptiMEM with cytokines (step 4d in preparation).
-
b.Record the cell counts from the sort and store tubes on ice.
-
c.In a sterile tissue culture hood, seed the 150 μL sorted cells in one well from a 48-wells plate (HSCs-plate) containing OP9 cells from step 4c in preparation (in total 300 μL per well).
-
a.
-
22.Virus Transduction.
 CRITICAL: Before performing the experiment, it is important to titrate your barcoding-containing lentivirus for the population of choice and determine the amount of virus required to achieve transduction efficiency that ensures the number of transduced cells per recipient does not exceed 10% of the complexity of the lentiviral barcode library, for an estimated barcode complexity of 80k as previously shown.4,6 Here, we titrated the virus using a dilution series and a multiplicity of infection (MOI) to achieve a transduction efficiency of 15–30% (Figure 5), to reduce the risk of multiple barcode integration, as previously described in Kristiansen et al.5
This step should be performed in an adequate cell culture hood using procedures approved for lentiviral transduction and adhering to the appropriate local safety and ethical protocols.
CRITICAL: Before performing the experiment, it is important to titrate your barcoding-containing lentivirus for the population of choice and determine the amount of virus required to achieve transduction efficiency that ensures the number of transduced cells per recipient does not exceed 10% of the complexity of the lentiviral barcode library, for an estimated barcode complexity of 80k as previously shown.4,6 Here, we titrated the virus using a dilution series and a multiplicity of infection (MOI) to achieve a transduction efficiency of 15–30% (Figure 5), to reduce the risk of multiple barcode integration, as previously described in Kristiansen et al.5
This step should be performed in an adequate cell culture hood using procedures approved for lentiviral transduction and adhering to the appropriate local safety and ethical protocols.-
a.Add the previously optimized amount of lentivirus to each well except for the un-transduced controls.
-
b.Gently rock the plate to disperse the virus.
-
c.Incubate in humidified incubator at 37°C and 5% CO2 incubator overnight.
-
d.Wash the cells in the morning.
-
i.Centrifuge plate at 350 g for 5 min at 4°C.
-
ii.Remove supernatant and wash the cells with 300 μL of FACS buffer, spin at 350 g for 5 min at 4°C,
-
iii.Remove supernatant and repeat the wash one more time. Centrifuge the plate at 350 g for 5 min, 4°C.
-
iv.Remove the supernatant. Add 300 μL of complete OptiMEM with cytokine and incubate in humidified, 37°C and 5% CO2 incubator.Note: Make sure to adhere to your specific safety rules regarding the handling of virus-transduced cells according to your local safety and ethical protocols.
-
i.
-
a.
-
23.Measure transduction efficiency (Day 3).
-
a.Collect 50 μL of the transduced cells and un-transduced controls into individual FACS tubes with 200 μL of FACs buffer.
-
b.Centrifuge samples at 350 × g for 5 min at 4°C and remove supernatant.
-
c.Re-suspend each sample in 150 μL staining buffer containing 7AAD.
-
d.Analyze GFP+ cells by FACS among live (7AAD-Neg) cells (Figure 5).
-
a.
-
24.Transfer cells to larger plate when confluent (approximately day 7–11).
-
a.Seed 50k OP9 cells in 6-well plates (Op9-plate) with 2 mL of complete optiMEM at the day of, or (>6 h) before transferring cells.
-
b.The following day, remove 1.3 mL of medium in Op9-plate (700 μL left in Op9-plate).
-
c.Homogenize the cells in the HSCs-plate by pipetting, and transfer cells (300 μL/well) to the Op9-plate from step 24b (in total 1 mL/well).
-
d.Rinse each well from the HSCs-plate 4 times with 250 μL of fresh complete optiMEM with cytokine concentration appropriate for a total volume of 2 mL, and transfer to Op9-plate. The final volume after transfer to Op9-plate is 2 mL per well.
-
a.
-
25.
Change half of the medium every 7 days to fresh complete optiMEM with cytokine.
Note: The cytokine concentration should appropriate for a total volume of 2 mL according to the table “Alternative 2- cytokine” in the “materials and equipment” section, without disturbing the cell layers.
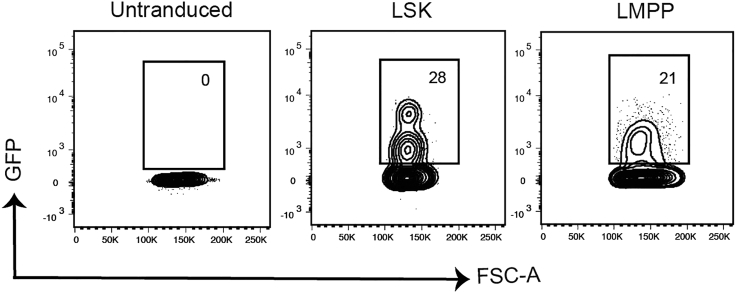
Figure 5.
Example of GFP transduction efficiency readout on day 3
Viable single LSK and LMPP cells are shown from un-transduced control (left) and transduced LSK and LMPP samples (middle and right respectively).
Barcode extraction, day 21 post transduction
In this step we recover the cell-specific barcodes. All steps are previously described in Kristiansen et al.5 Differentiation kinetics is unique for each progenitor and can additionally be affected by the virus transduction. It is recommended to optimize the readout time based on the specific population assayed since readout time point can vary. Here, we sort LSK output cells at day 16 and day 21 and LMPP at day 8 and day 16 to ensure discovery of clonal output into multi-lineages with different differentiation kinetics.
-
26.Cell sorting and lyses.
-
a.Transfer cells into 3 mL FACS tubes and centrifuge at 350 × g for 5 min at 4°C.
-
b.Resuspend cells in FACS buffer containing FC-block (1:100) and incubate for 5 min in 4°C.
-
c.Stain cells according to the table “Alternative 2-antibody cocktail” in the materials and equipment, incubate 45 min in darkness at 4°C and gentle shaking.
-
d.Wash cells in FACS-buffer at 350 g, 8 min.
-
e.Re-suspend cells in FACS-buffer containing 7AAD (1:200).Note: Our staining suggestion was sorted on AriaIII according to configuration in “materials and equipment”.
-
f.The FACS gating strategy for sorting of Megakaryocyte, Erythroid, myeloid (granulocyte and Monocyte/Macrophage) and Lymphoid (B) cells are shown in (Figure 6).Note: For small number of cells, sort directly into lysis buffer.
-
g.Sort GFP+ cells into “non-stick tubes “(low-binding surface) containing 500 μL of FACS buffer. Store at −80°C as cell pellet for a maximum of 7 days before lysing.
-
a.
-
27.Extraction of genomic DNA.
-
a.Prepare lysis buffer according to table lysis buffer in the “materials and equipment”.
-
b.Cell pellets are lysed to extract genomic DNA in 100 μL cell lysis buffer for 2 h at 56°C, followed by 10 min at 95°C to inactivate proteinase K. Store at −20°C or continue with step 28.
-
a.
-
28.Library preparation and barcode sequencing.
-
a.Purify genomic DNA using Ampure XP beads in 1:1 ratio according to manufacturer's protocol (Beckman Coulter).
-
b.Measure genomic DNA concentration using a high sensitivity method e.g., the Qubit dsDNAhs Assay Kit according to manufacturer's protocol (Thermo Fisher Scientific).
-
c.Divide up to 40 ng DNA from each sample into two technical replicate PCR reactions (20 ng each).Note: Use the appropriate primers and a high-fidelity DNA Taq polymerase (e.g., Q5 Taq DNA polymerase) for amplification of the barcode fragment (Figure 4). PCR reaction master Mix and PCR cycling is described in “materials and equipment”. Following this step, the DNA can be stored at −20°C.
-
d.Purify the amplified fragments (155 bp) using Ampure XP beads 1:1.5× ratio according to the manufacturer’s recommendations (Beckman Coulter).Note: Here the fragments can be stored at −20°C.
-
e.Measure DNA concentration using a high sensitivity method e.g., the Qubit Fluorometer and the Qubit dsDNA HS Assay Kit.Optional: A second step of PCR amplification can be performed to add multiplexing adaptors, depending on the design of the PCR primers and the sequencing platform used.
-
f.Run second PCR according to the manufacturer’s recommendations (Thermo Fisher Scientific, InvitrogenTM).Note: PCR reaction master Mix and PCR cycling condition are described in “materials and equipment”. The PCR product can be stored at −20°C.
-
g.Purify the amplified fragments (225 bp) using Ampure XP beads 1:1.5× ratio as previously described in “11d”. The fragments can be stored at −20°C.
-
h.Quantify amplicons (size and concentration) using the Bioanalyzer HS DNA Analysis Kit.Note: A product of the correct size without contaminating primer dimer is critical for successful sequencing. The presence of primer dimers can be removed through an additional round of Ampure XP bead purification.
-
i.Pool equivalent amounts of amplified and indexed barcode libraries for sequencing according to the instructions for the chosen method for sequencing.Note: Here we used the Illumina MiSeq instrument and the v3 high output kit (150 cycles).
-
a.
Statistical analysis
The aim of the data analysis is to generate a list of reliable barcodes for each sorted sample, which will be combined with barcode read frequency, reflecting the abundance of each clone within the population. To enable rapid analysis of the data generated using our protocol, we have developed a Python package called Bartide. The package contains modules tailored to streamline three key challenges viz., barcode extraction, sequencing error correction and cross-sample comparison.
-
29.
To install Bartide you need to have Python version 3.9 or upward. Use the following command to install Bartide.
>pip install bartide==0.3.2
-
30.
Barcodes are extracted from the sequencing reads using Bartide’s BarcodeExtractor class. This class is provided two input files, one for each end of paired-end sequencing, in FASTQ format.
Note: This class is designed to automatically extract the barcodes assuming that the barcodes are of the same lengths, they span the same position in the reads, and the flanking sequence is constant. This is achieved by first summarizing the nucleotide composition at each position for all the reads (or a sample of reads). The flanking sequence will be dominated by a single nucleotide while the barcode should have variable base composition. This pattern is used to identify the position of barcodes and the flanking primer sequence. This behaviour can be overridden by providing the barcode length and the flanking primer sequence to BarcodeExtractor. Below we show an example of how to call the BarcodeExtractor class and perform automatic flank identification.
>import bartide
>extractor = bartide.BarcodeExtractor(
‘sample1_read1.fastq.gz’,
‘sample1_read2.fastq.gz’
)
>extractor.identify_flanks()
Alternatively, users can provide the flanking sequence and the barcode lengths manually as shown below:
>extractor = bartide.BarcodeExtractor(
‘sample1_read1.fastq.gz’,
‘sample1_read2.fastq.gz’,
left_flank='GTAGCC',
right_flank='AGATCG',
barcode_length=27
)
-
31.
Once the flank sequences and barcode length are determined, they are stored as extractor.leftFlank, extractor.rightFlank and extractor.barcodeLength. Now the barcodes can be extracted, and their frequency counted.
Note: The BarcodeExtractor class will compare the barcode sequence and its reverse complementary sequence from the other pair of the sequenced read. By default, there should not be more than 3 mismatches between the two sequences otherwise the extraction fails for that read. Users can change the maximum allowed mismatch value by using the max_dist parameter when calling BarcodeExtractor.
The actual extraction of barcodes is triggered by the following command:
>extractor.count_barcodes()
Users can access these uncorrected barcodes with the following command:
>print (extractor.rawCounts)
This prints the barcodes and their frequencies as shown below:
TTGTAGGGGTGTGTTCTACCGGTAATT 2843
GTGCTGGTAATGTGGGCGACGGTGGGG 913
TTGGTGAAGCATAGTTCCGTGATTGAA 909
TTCCATGACGTTAAATACCTCCTTATA 723
ATCTGGCGTCCAGCAGATATTAGTTTT 717
...
AAGTTACATGCCGCAAAGGGTTCTTTG 1
AAGGATGAATGACAAGGTGCTAGCCAT 1
GGTACAAGGCGGGATTACCATGCATTG 1
GTGCTGGAAATGTGGGCGACGGTGGGG 1
AAGTCACATGCCGCAAAGTGTCCATTG 1
-
32.
Next, the list obtained above may still contain barcodes that harbor sequencing errors. We assume that a barcode contains error(s) if it has less than three nucleotide differences with a higher abundance barcode. Since the pairwise comparison of all the barcodes can be computationally prohibitive, we use the approximate nearest neighbor detection library ‘nmslib’ to efficiently identify similar barcodes.
Note: If an erroneous barcode is found, its frequency is added to the barcode with the nearest match. This functionality is implemented in the SeqCorrect class. Users can obtain the corrected barcode list (in the same format as the uncorrected list above), by running the following command:
>corrector = bartide.SeqCorrect()
>corrector.run(extractor.rawCounts)
The corrected list of barcodes is stored under corrector.correctedCounts. These barcodes can then be saved in CSV format table as shown below:
>corrector.save_to_csv(‘barcodes_freq_sample1.csv’)
The SeqCorrect class will by default, remove any barcode with a frequency of less than 20, as suggested previously 5. This behavior can be overridden by changing the value of the min_counts parameter when calling SeqCorrect.
-
33.
Once the corrected barcode frequencies are saved for all the samples, they can be compared using the BarcodeAnalyzer class.
Note: This class is initialized by providing the name, with full path, of the directory wherein all the CSV files were saved. the following command illustrates this:
>analyzer = bartide.BarcodeAnalyzer(‘barcodes_dir’)
This will lead to aggregation of all the barcodes across all the samples that can be accessed from a single table stored under analyzer.barcodes. Users can perform all the custom downstream analysis using this table as the starting point.
-
34.
Bartide provides four essential plots that allow users to easily identify how the barcodes are shared across the sample.
Note: The first plot is the ‘Upset’ that shows all the combinations of samples and the number of barcodes that overlap between them. This plot, compared to Venn diagrams, allows easy visualization of the overlaps and non-overlaps of barcodes. Please note that when using upset plots, we only look at the unique number of barcodes found in each of the samples and not their frequencies.
>analyzer.plot_upset()
Sometimes due to large differences in the number of barcodes captured, it might be difficult to easily identify the similarity or differences between the samples. To solve this, rather than using the absolute number of barcodes in a sample, the percentage overlap of barcodes from a sample with all other samples is used. This allows the barcodes from a sample to be defined in terms of proportions and may allow insights into sample similarity that is otherwise difficult to discern using absolute frequencies. The following command shows the proportions in the form of a stacked barplot:
>analyzer.plot_stacked()
An alternative way to deal with the situation wherein the absolute number of unique barcodes is quite different across the samples is to perform normalization by dividing the overlap value by the sum of the total barcodes from the two samples. The resulting normalized values can be visualized in the form of a heatmap.
>analyzer.plot_overlap_heatmap()
In all the above three plotting functions, we do not use the frequencies of the barcodes, which are indicative of how dominant a particular barcode is in the samples. A weighted overlap of barcodes is calculated between two samples as following:
Wherein, S is a column-sum normalized matrix of samples (columns) and barcodes (rows) containing barcode frequencies, i and j are two samples, b is a barcode in a set of barcodes B that are present in either of the two samples or both. These overlap values are then plotted in the form of a heatmap using the following function:
>analyzer.plot_weighted_heatmap()
To save the images generated by the functions above, users can pass a value (path and name of the file where to save) to the save name parameter.
Expected outcomes
Alternative 1 (switch culture)
Figure 3 depict representation of data from our recent study Safi et al.,1 Figures 3B and 3C show representative gating strategy in cultures from a single LT-HSC. The graph bar in Figure 3D shows that 52% of the clones derived from single LT-HSCs generated oligopotent progeny (2% Myeloid-B-Erythroid, 26% Myeloid-Erythroid, 23% Myeloid-B) while the remaining clones generated unilineage output (31% GM, 5.5% B and 2% ER). In contrast 70% of LMPPs clones generated unilineage, B cell output.
Alternative 2 (barcoding protocol)
Figures 6 and 7 display an example of data from the barcoding culture system. Figure 6 shows that LSK cells can differentiate to all lineages (Myeloid (My), Macrophage (MΦ), B, Megakaryocyte (MK) and Erythroid (Er)) using our in vitro co-culture system following 21-days. In contrast the LMPP population only produce B and Myeloid cells. Figure 7 demonstrates the qualitative overlap between barcodes derived from potential B, My (My + MΦ) and Er/MK (Er + MK) in LSK or LMPP populations. In LSK, 2% of the barcodes are present in all populations (My, Er/MK, and B), while 35% overlap between multiple lineages (My-Er-MK (32%), My-B (2%) and B-Er/MK (1%). The remaining barcodes marks unilineage differentiation (My (41%), MK (18%) and B (4%)). In LMPPs, 29% of the barcodes are present in both B and MY cells, while the remaining 21% or 40% were unique to either My or B respectively.
Figure 7.
Multipotent progenitors of LSK cells revealed by barcoding method, the number of unique barcodes that are present in individual samples and all combinations thereof
(A and B) Each bar represents the barcode combination as depicted by the filled dark circles under it. The horizontal bars in the lower left of the figure show total number of unique barcodes detected (after error correction) in each of the three samples (A) LSK and (B) LMPP.
(C and D) Heat maps showing the normalized shared barcode abundances across samples. The color map captures the values in the range of 0 and 1, wherein 1 means perfect overlap of samples and 0 is no overlap (C) LSK and (D) LMPP.
(E and F) Each stacked bar shows the percentage of barcodes from a sample (as shown in the x-axis) that are shared with other samples (as shown in the legend above the bars). For all the three samples here, the largest percentage is not shared with other samples meaning that those barcodes are not shared with other samples (E) LSK and (F) LMPP.
Limitations
In general, these protocols are designed to assess the differentiation capacity of the earliest hematopoietic stem and progenitor cells in-vitro. Compared to conventional in vivo assays in vitro assays have both financial and ethical benefits, reducing the need for animal experiments. However, there are some limitations and possible sources of variation of the described protocols.
First, this protocol is designed to measure differentiation to the three major lineage trajectories of hematopoiesis (lymphoid (B cells), myeloid and Megakaryocyte/Erythroid). However, it is not possible to differentiate clones to all cells of the blood system. E.g., T cell differentiation requires optimization including co-culture on OP9-DL17.7
Second, healthy male or female mice at age of 8–12 weeks were used to develop the protocol. Adjustments might be needed when performing experiments on cells from mice at other stages of ontology, strains, or disease models.
Third, the quantity of sorted cells is a restriction of the switch culture protocol. Due to many sensitive and laborious steps, it is challenging to work with more than 96 cells at a time. Therefore, we present and alternative less time-consuming protocol where barcoding methods are utilized for higher throughput and easier handling. Compared to the switch culture, the barcoding method has the advantage of being able to prepare thousands of cells in a single experiment, which make it easier to compare several different populations simultaneously.
The barcoding protocol gives higher throughput and requires less handling. The differentiation protocol is sensitive and accurate in generating clonal multilineage differentiation when assaying HSC-containing LSK cells (Figure 6A), while lineage restriction is efficiently demonstrated from lineage-committed progenitors (Figure 6B).
Troubleshooting
Problem 1
No B cells are identified from a population with expected B cell differentiation potential, e.g., LMPP.
Note: We recommended that you include bulk sorted LMPP cells as positive control for B lineage output.
Potential solution
If OP9 cells overgrow, they will differentiate making them unable to support B-cell differentiation. Make sure OP9 cells never grow beyond 80% confluence (Figure 1B). To ensure that OP9 cells recover after thawing and secrete the required cytokines, we suggest they are passaged at least 2 times before the experiment.
The timing of read out might be too early as the differentiation kinetics varies between different populations, e.g., the HSCs generate B cells following 3 weeks co-culture, while LMPP cells begin to produce B cells already after 8 day of co-culture with OP9 cells.
Finally, be careful to ensure the correct concentrations of 2-mercaptoethanol and cytokines as well as to control FBS quality (pre-selected batches).
Problem 2
Not all expected differentiated populations are present at the day of readout.
Potential solution
The differentiation kinetics of different progenitor cells and different lineages differs. Myeloid cells differentiate fast, while Erythroid cells, and especially B cells occur at later time-points as previously reported in Upadhaya et al.8 It is likely that different lineage output should be analyzed at different readout time points. Additionally, in in-vitro culture systems differentiation kinetics can differ depending on whether you’re working with single (slower differentiation) or bulk cells (faster differentiation). We recommend optimizing readout time beforehand and sort a few control wells for each population that can be analyzed for presence of all expected populations prior to the actual experiment is run. Furthermore, following 24 days of co-culture, cells started to lose viability, therefore, we recommended reading out cells prior to 24 days of co-culture.
Problem 3
There is risk of contamination due to long culture process and several handling steps.
Potential solution
It is important to work with sterile procedures and sterilize hoods and instruments.
Problem 4
In the barcoding protocol, Individual cells have multiple barcodes.
Potential solution
To reduce the risk of multiple barcode integration, decrease the transduction efficiency by lowering the (MOI). It is recommended to keep transduction efficiency below 30% in multiple reports.5,4
Problem 5
In the barcoding protocol, false positive barcode overlaps readouts.
Potential solution
Barcode overlap at readout is critical for kinship analysis of differentiation lineage cells. To avoid false positive overlap readout, it is important to control that the same barcode does not enter more than one cell. This can be done by comparing the filtered barcode list from two technical replicates of LSK cells transduced in the same well and divide in two as previously explained in Naik et al.4 Since the short virus incubation time is not likely to permit HSC division, any overlap between the two replicates can be considered as noise. The current protocol is designed as previously describe in Kristiansen et al.5
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Göran Karlsson (goran.karlsson@med.lu.se).
Materials availability
This study did not generate new unique regents.
Acknowledgments
We thank Dr. Joan Yuan, Dr. Trine A Kristiansen, and Abdul Ghani Alattar for providing us with the Lentiviral Barcode Library and for valuable input and discussion for experimental design. We would also like to thank Anna Fossum and Zhi Ma at the FACS Core facility at Lund Stem Cell Center for expert support in flow cytometry. This work was supported by grants from the Swedish Cancer Society, the Ragnar Söderberg Foundation, the Knut and Alice Wallenberg Foundation, the Swedish Research Council, the Swedish Society for Medical Research, and the Swedish Childhood Cancer Foundation.
Author contributions
G.K., F.S., and C.B. conceived, designed, and supervised the study; F.S. designed and performed the experiments with contributions from E.E., L.G.U., and A.S.C. for validation; P.D. designed and performed the bioinformatics analyses. F.S. and G.K. analyzed and interpreted data; F.S. wrote the manuscript and prepared the figures with contributions from P.D. and input from all authors.
Declaration of interests
The authors declare no competing interests.
Data and code availability
A Python package called Bartide was generated during this study. The source code of the package can be accessed here: https://github.com/parashardhapola/bartide. An archived version (0.3.2) of the source code used in this protocol is available here: https://doi.org/10.5281/zenodo.7398381.
References
- 1.Safi F., Dhapola P., Warsi S., Sommarin M., Erlandsson E., Ungerbäck J., Warfvinge R., Sitnicka E., Bryder D., Böiers C., et al. Concurrent stem- and lineage-affiliated chromatin programs precede hematopoietic lineage restriction. Cell Rep. 2022;39:110798. doi: 10.1016/j.celrep.2022.110798. [DOI] [PubMed] [Google Scholar]
- 2.Bystrykh L.V., Belderbos M.E. Clonal analysis of cells with cellular barcoding: when numbers and sizes matter. Methods Mol. Biol. 2016;1516:57–89. doi: 10.1007/7651_2016_343. [DOI] [PubMed] [Google Scholar]
- 3.Bystrykh L.V., de Haan G., Verovskaya E. Barcoded vector libraries and retroviral or lentiviral barcoding of hematopoietic stem cells. Methods Mol. Biol. 2014;1185:345–360. doi: 10.1007/978-1-4939-1133-2_23. [DOI] [PubMed] [Google Scholar]
- 4.Naik S.H., Schumacher T.N., Perié L. Cellular barcoding: a technical appraisal. Exp. Hematol. 2014;42:598–608. doi: 10.1016/j.exphem.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Kristiansen T.A., Doyle A., Yuan J. Lentiviral barcode labeling and transplantation of fetal liver hematopoietic stem and progenitor cells. Bio. Protoc. 2017;7:e2242. doi: 10.21769/BioProtoc.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R., Neff N.F., Quake S.R., Weissman I.L. Tracking single hematopoietic stem cells in vivo using high-throughput sequencing in conjunction with viral genetic barcoding. Nat. Biotechnol. 2011;29:928–933. doi: 10.1038/nbt.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt T.M., Zúñiga-Pflücker J.C. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 8.Upadhaya S., Sawai C.M., Papalexi E., Rashidfarrokhi A., Jang G., Chattopadhyay P., Satija R., Reizis B. Kinetics of adult hematopoietic stem cell differentiation in vivo. J. Exp. Med. 2018;215:2815–2832. doi: 10.1084/jem.20180136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A Python package called Bartide was generated during this study. The source code of the package can be accessed here: https://github.com/parashardhapola/bartide. An archived version (0.3.2) of the source code used in this protocol is available here: https://doi.org/10.5281/zenodo.7398381.