Abstract
Background
Visit-to-visit body weight variability (BWV), pulse rate variability (PRV), and blood pressure variability (BPV) have been respectively linked to multiple health outcomes. The associations of the combination of long-term variability in physiological measures with mortality and epigenetic age acceleration (EAA) remain largely unknown.
Methods
We constructed a composite score of physiological variability (0-3) of large variability in BWV, PRV, and BPV (the top tertiles) in 2006/2008–2014/2016 in the Health and Retirement Study (HRS) and 2011–2015 in the China Health and Retirement Longitudinal Study (CHARLS). All-cause mortality was documented through 2018. EAA was calculated using thirteen DNA methylation-based epigenetic clocks among 1047 participants in a substudy of the HRS. We assessed the relation of the composite score to the risk of mortality among 6566 participants in the HRS and 6906 participants in the CHARLS by Cox proportional models and then investigated its association with EAA using linear regression models.
Results
A higher score of variability was associated with higher mortality risk in both cohorts (pooled hazard ratio [HR] per one-point increment, 1.27; 95% confidence interval [CI], 1.18, 1.39; P-heterogeneity = 0.344), after adjustment for multiple confounders and baseline physiological measures. Specifically, each SD increment in BWV, PRV, and BPV was related to 21% (95% CI: 15%, 28%), 6% (0%, 13%), and 12% (4%, 19%) higher hazard of mortality, respectively. The composite score was significantly related to EAA in second-generation clocks trained on health outcomes (e.g., standardized coefficient = 0.126 in the Levine clock, 95% CI: 0.055, 0.196) but not in most first-generation clocks trained on chronological age.
Conclusions
Larger variability in physiological measures was associated with a higher risk of mortality and faster EAA.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02674-w.
Keywords: Body weight variability, Pulse rate variability, Blood pressure variability, Mortality, Epigenetic aging
Background
Global population aging forecasts an increasing burden of disease and disability on public health [1–3]. Aging is viewed as a gradual and progressive deterioration in the integrity of biological systems and can be indicated by molecular- and cellular-level changes [4]. Specifically, the loss of ability in homeostasis maintenance is a key feature of biological aging [5], potentially through the hypothalamus-related regulatory axes [6].
Older adults tend to have fluctuating physiological measures [7, 8], and previous studies have linked the long-term variabilities in these measures to subsequent risk of multiple aging-related health outcomes, including frailty [9, 10], cardiovascular diseases [11, 12], dementia [13–15], and mortality [16]. For example, a meta-analysis [16] showed that each SD increment in BPV was associated with a 15% higher risk of all-cause mortality. However, the overall associations of long-term variabilities in major physiological measures with aging remained unclear. First, most previous studies focused on one specific type of physiological measure, while aging is often accompanied by homeostatic imbalances in multiple systems [4], and the combined relation of these variability measures to mortality warrants investigation across populations. Second, most previous studies utilized relatively short-term measurements of physiological measures, and the relation of long-term variability in physiological measures with mortality has been less explored. Moreover, the underlying mechanism remained unclear. Although epigenetic clocks have been suggested to capture the complexity between aging and homeostasis maintenance [17] and reflect biological aging [18–21], few studies have examined their associations with variabilities in physiological measures.
Therefore, we hypothesize that the long-term variability in multiple physiological measures could be linked to accelerated aging. To assess the relation of the combination of BWV, PRV, and BPV with mortality and epigenetic accelerated aging (EAA), we leveraged two nationally representative prospective studies, the Health and Retirement Study (HRS) in the USA and the China Health and Retirement Longitudinal Study (CHARLS). In addition, the HRS 2016 Venous Blood Study (VBS) provided a unique opportunity to analyze a panel of well-constructed epigenetic clocks based on high-quality DNA methylation data [22].
Methods
Study design and population
This study was based on the Health and Retirement Study (HRS) [23] and the China Health and Retirement Longitudinal Study (CHARLS) [24], two national cohort studies with similar designs [25, 26].
In the HRS, participants aged 50 or older were recruited and revisited biennially for collection of sociodemographic, lifestyle, and clinical information. Half of a randomly selected sample received physical examinations in 2006, 2010, and 2014 and the other half in 2008, 2012, and 2016 [25]. DNA methylation assays were conducted in a representative subsample of the HRS 2016 Venous Blood Study (VBS) with comparable distributions of age, sex, education, and race/ethnicity to the entire HRS 2016 sample born before 1960 [27]. For example, the HRS 2016 sample had a mean age of 69.0 years and 59.3% female, and the corresponding values were 68.7 years and 58.0% in the VBS. The HRS was approved by the Health Sciences and Behavioral Sciences Institutional Review Board at the University of Michigan (HUM00061128).
The CHARLS recruited participants aged 45 years or older in 2011 and revisited them in 2013, 2015, and 2018. Face-to-face interviews and physical examinations were administered to all participants at each wave. The CHARLS was approved by the Ethics Committee of Pecking University (00001052-11, 014).
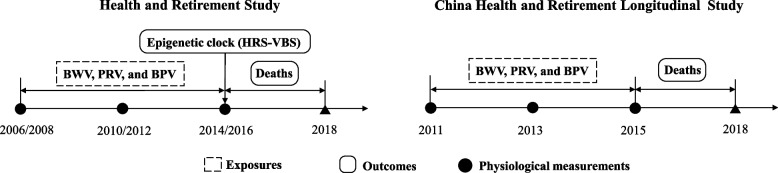
We conducted two primary analyses (Fig. 1 and Additional file 1: Fig. S1). First, we assessed the relations of a composite score of variability in physiological measures to mortality, including 6566 HRS participants and 6906 CHARLS participants who (1) received three measurements of body mass index (BMI), pulse rate, and blood pressure in 2006–2016 (HRS) or 2011–2015 (CHARLS) and (2) were followed up to the next wave or had death information during follow-up. Among the HRS-VBS participants who had high-quality DNA methylation data in 2016 (N = 1047), we investigated the association between the composite score and EAA.
Fig. 1.
Study design. BWV, body weight variability; PRV, pulse rate variability; BPV, blood pressure variability; BMI, body mass index
Variability in body weight, pulse rate, blood pressure, and composite score
Body weight, height, pulse rate, and blood pressure in the HRS (2006–2014 or 2008–2016) and the CHARLS (2011–2015) were measured following similar protocols. Body weight was measured using an electronic weighing scale without shoes and with light clothing, and height was measured by having the respondent stand against a wall without shoes [28, 29]. BMI was calculated as weight in kilograms divided by height in meters squared. Pulse rate and blood pressure were each assessed three times, 45 to 60 s apart, respectively, in a seated position, by trained interviewers using an automated sphygmomanometer [28, 30]. The average values of the last two valid measurements were used at each wave.
Visit-to-visit BWV, PRV, and BPV were calculated as the coefficients of variation (CV), i.e., quotient of standard deviation (SD) and mean, of BMI, pulse rate, and systolic blood pressure (SBP), respectively. We used systolic blood pressure variability (SBPV) to represent BPV because it had stronger associations with adverse health outcomes than diastolic blood pressure (DBP) [31, 32]. We also used DBPV as an alternative BPV measure in the sensitivity analysis.
To reflect the overall variability in physiological measures, we constructed a composite score following the strategy of a prior study [33]. The highest tertiles of BWV, PRV, and BPV were each assigned 1 point, and the middle and lowest tertiles were assigned 0 point. We took the sum of the three binary indicators as the composite score (range: 0–3), and a higher score represented greater intraindividual instability in the physiological measures.
Ascertainment of mortality
All-cause mortality was documented from the latest physiological measurements (2014 or 2016 in HRS and 2015 in CHARLS) to the end of follow-up (2018). The HRS and CHARLS adopted similar strategies to identify deaths using data linkage from the population registry and through interviews with informants or knowledgeable others [34, 35].
Epigenetic clock and epigenetic age acceleration
In the HRS-VBS, DNA methylation was assayed using the Infinium Methylation EPIC BeadChip that covered over 850,000 CpG sites (test-retest reliability correlation > 0.97 for all CpG sites) [36]. Thirteen epigenetic clocks were constructed based on DNA methylation data. Nine first-generation clocks (Horvath 1, Hannum, Horvath 2, Lin, Weidner, VidalBralo, Yang, Bocklandt, and Garagnani) were trained on chronological age, and four second-generation clocks (Levine, Zhang, GrimAge, and DunedinPoAm38) were trained on health-related outcomes [22, 37]. The thirteen epigenetic clocks [38–50] combined information for a number of CpGs (typically 100–500) to produce indicators of epigenetic aging. For example, Horvath 1 used 353 CpGs selected by the elastic net regression model [38]. Missing beta methylation values were imputed with the mean value of the given probe across all samples [36]. More information can be found in the HRS-VBS protocol [36].
We defined EAA following the strategy in a previous study in the HRS-VBS [22]: each clock value was regressed on chronological age in 2016 and the standardized residual reflected EAA. A larger value of the standardized residual indicated faster biological aging when the chronological age was fixed [51].
Other covariates
In HRS and CHARLS, demographic, lifestyle, and clinical factors were collected using questionnaires at baseline, including age, gender defined by self-identity, education level (high school degree level, yes/no), marital status (married or not), smoking status (never/former/current), alcohol consumption (never/former/current), household income (in quartiles for HRS and < 9999 yuan/≥ 10,000 yuan/missing for CHARLS), physical activity engagement (> 1 time per week/1–3 times per month/never for HRS and any/never/missing for CHARLS), and self-reported diabetes mellitus, heart disease, and stroke. In the HRS, race (white/black/others) was self-reported. In the CHARLS, residence (rural/urban) was identified from the registry.
Statistical analyses
Baseline characteristics of the 6566 HRS participants, 6906 CHARLS participants, and 1047 HRS-VBS participants were presented in mean ± standard deviation (SD) for continuous variables and number (percentage) for categorical variables.
In the primary analyses, we assessed the association between the composite score of variability in physiological measures and risk of mortality using Cox proportional hazard models. Person-time was calculated from the latest physiological measurement to the year of death or the end of follow-up, whichever came first. We sequentially adjusted the hazard ratios (HRs) and 95% confidence intervals (CIs) for baseline age, gender, race (for HRS only), residence (for CHARLS only), education level, household income, marital status, smoking status, alcohol consumption, physical activity engagement, baseline BMI, baseline pulse rate, and baseline blood pressure. The estimates from the two cohorts were pooled using fixed-effect models when no significant heterogeneity was detected. Missing values for all continuous covariates (< 2%) were imputed by means and categorical variables by the most populated categories, except for household income and physical activity engagement in the CHARLS (using a missing category). We used linear regression models to evaluate the associations between the composite score and EAA indicated by the 13 epigenetic clocks in the HRS. Linear trend was tested by assigning the median value of each category and including it as a continuous variable in the models. To address multiplicity, we adjusted the P-values using the Benjamini-Hochberg (B-H) methods in the analysis of the composite score and EAA [52].
In the secondary analysis, we assessed the individual associations of BWV, PRV, and BPV with mortality and EAA, respectively. The adjustment was the same as the primary analysis, and baseline BMI, baseline pulse rate, and baseline blood pressure were separately adjusted for the corresponding exposures.
We performed multiple sensitivity analyses by (1) adjusting the models for baseline health conditions (diabetes mellitus, heart disease, and stroke); (2) alternatively using standard deviation (SD) and variation independent of the mean (VIM) to represent BWV, PRV, and BPV; (3) using DBPV in place of SBPV; (4) constructing the composite score using the medians of BWV, PRV, and BPV as cutoffs rather than the top tertiles; (5) alternatively assigning 0, 1, and 2 to the increasing tertiles of variability indicators to construct a new composite score ranging from 0 to 6 and repeated the primary analysis; and (6) adjusting the models for cell-type proportions (the percentages of neutrophil, basophilic granulocyte, eosinophilic granulocyte, mononuclear granulocyte, and leukomonocyte in the total count of white blood cells) in the analysis of EAA [53, 54].
Statistical analyses were performed using R 4.1.0. We reported two-sided P values and 95% confidence intervals (CIs) throughout, and tests with P < 0.05 were considered statistically significant.
Results
Participant characteristics at baseline
Baseline characteristics of the study population in the HRS cohort (N = 6566), HRS-VBS (N = 1047), and CHARLS (N = 6906) were presented in Table 1. Compared with HRS participants, adults in the CHARLS were younger (62.5 years vs. 73.0 years), fewer in females (54.2% vs. 60.7%), less educated, more likely to be current smokers, less likely to be current alcohol consumers, and had smaller BWV, PRV, and BPV. Among the 1047 HRS-VBS participants, 59.6% were female, and 87.3% were White/Caucasian. The mean (SD) age of the HRS-VBS participants was 77.0 (5.5) years old, and the means of long-term BWV, PRV, and BPV were 4.9%, 9.0%, and 9.3%, respectively. Descriptive statistics of the 13 epigenetic clocks and EAA were displayed in Additional file 1: Table S1.
Table 1.
Baseline characteristics of the study participants
| Characteristicsa | HRS | CHARLS | |
|---|---|---|---|
| HRS cohort | HRS-VBS | ||
| N | 6566 | 1047 | 6906 |
| Age, years, mean ± SD | 73.0 ± 9.1 | 77.0 ± 5.5 | 62.5 ± 9.0 |
| Female, N (%) | 3988 (60.7) | 624 (59.6) | 3741 (54.2) |
| Having high school degree level, N (%) | 5594 (85.2) | 881 (84.1) | 633 (9.2) |
| Married, N (%) | 4093 (62.4) | 652 (62.3) | 5648 (81.8) |
| Caucasian, N (%) | 5420 (82.5) | 914 (87.3) | – |
| Rural resident, N (%) | – | – | 4792 (69.4) |
| Smoking status, N (%) | |||
| Current | 602 (9.2) | 68 (6.5) | 1849 (26.8) |
| Former | 2944 (45.0) | 519 (49.6) | 1148 (16.6) |
| Never | 3013 (46.2) | 460 (43.9) | 3909 (56.6) |
| Alcohol consumption, N (%) | |||
| Current | 2522 (38.5) | 382 (36.5) | 2220 (32.2) |
| Former | 1080 (16.5) | 151 (14.4) | 817 (11.8) |
| Never | 2944 (45.0) | 514 (49.1) | 3869 (56.0) |
| Stroke, N (%) | 609 (9.3) | 118 (11.3) | 263 (3.8) |
| Heart diseases, N (%) | 1963 (30.0) | 379 (36.2) | 1230 (18.2) |
| Diabetes, N (%) | 1655 (25.2) | 299 (28.6) | 679 (10.1) |
| Body mass index, kg/m2, mean ± SD | 29.2 ± 5.6 | 29.0 ± 5.6 | 23.7 ± 4.2 |
| Systolic blood pressure, mmHg, mean ± SD | 129.3 ± 19.1 | 130.1 ± 20.0 | 128.0 ± 20.2 |
| Pulse rate, bpm, mean ± SD | 68.8 ± 10.9 | 67.8 ± 10.6 | 73.9 ± 10.8 |
| BWV, %, mean ± SD | 5.3 ± 4.6 | 4.9 ± 4.4 | 4.2 ± 5.2 |
| BPV, %, mean ± SD | 9.3 ± 5.5 | 9.3 ± 5.3 | 8.7 ± 5.6 |
| PRV, %, mean ± SD | 9.1 ± 5.8 | 9.0 ± 5.8 | 8.9 ± 5.2 |
| Household income, N (%) | |||
| Quartile 1 | 1643 (25.0) | 262 (25.0) | – |
| Quartile 2 | 1642 (25.0) | 262 (25.0) | – |
| Quartile 3 | 1640 (25.0) | 261 (25.0) | – |
| Quartile 4 | 1641 (25.0) | 262 (25.0) | – |
| 0–9999 yuan | – | – | 1909 (27.6) |
| ≥ 10,000 yuan | – | – | 2265 (32.8) |
| Missing | – | – | 2732 (39.6) |
| Physical activity, N (%) | |||
| ≥ 1 time/week | 2305 (35.1) | 350 (33.4) | – |
| < 1 time/week | 3941 (60.1) | 647 (61.8) | – |
| Never | 314 (4.8) | 50 (4.8) | – |
| Any | – | – | 1922 (27.8) |
| Never | – | – | 1499 (21.7) |
| Missing | – | – | 3485 (59.5) |
HRS Health and Retirement Study, CHARLS China Health and Retirement Longitudinal Study, HRS-VBS Health and Retirement Study - Venous Blood Study, BMI body mass index, Q quartile, BWV body weight variability, BPV blood pressure variability, PRV pulse rate variability
aValues are described as mean ± SD and percentage (%) for continuous and categorical variables, respectively
Associations between the composite score of variability and mortality and epigenetic age acceleration
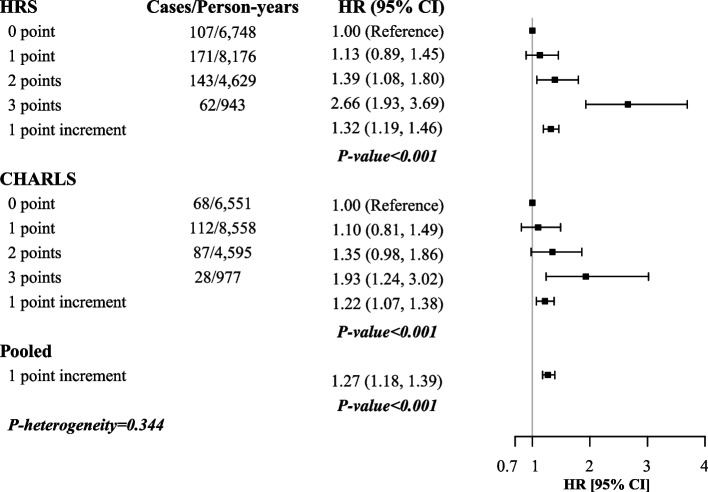
A total of 778 participants died during follow-up, including 483 of the 6566 HRS participants (20,496 person-years) and 295 of the 6906 CHARLS participants (20,681 person-years). The association between the composite score of variability in physiological measures and mortality was observed in both cohorts (Fig. 2). Adjusted for multiple confounders and baseline levels of these physiological measures, each point increment of the composite score was related to a 27% (95% CI: 18%, 39%, P-heterogeneity = 0.344) higher risk of mortality. The corresponding HRs (95% CIs) were 1.32 (1.19, 1.46) in the HRS and 1.22 (1.07, 1.38) in the CHARLS. Compared with a variability score of 0, a score of 3 (co-existence of large BWV, PRV, and BPV) was associated with the highest risk of mortality (HR = 2.66, 95% CI: 1.93, 3.69 in the HRS and HR = 1.93, 95% CI: 1.24, 3.02 in the CHARLS). Detailed results could be found in Additional file 1: Table S2.
Fig. 2.
Multivariable adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of mortality associated with the composite score of variability in the HRS and CHARLS. HRS, Health and Retirement Study; CHARLS, China Health and Retirement Longitudinal Study; HR, hazard ratio; CI, confidence interval. Models were adjusted for age (continuous), gender (female/male), education (high school degree level, yes/no), marriage status (yes/no), residence (rural/urban) in the CHARLS, race (White/Black/others) in the HRS, drinking status (current/ever/never), smoking status (current/ever/never), physical activity (> 1 time per week/1–3 times per month/never in the HRS and any/never/missing in the CHARLS), household income (quartiles in the HRS and ≤ 9999 yuan/≥ 10,000 yuan/missing in the CHARLS), body mass index, systolic blood pressure, and pulse rate (all continuous) in 2014/2016 (HRS) or in 2015 (CHARLS). Fixed effect model was used to pool the results
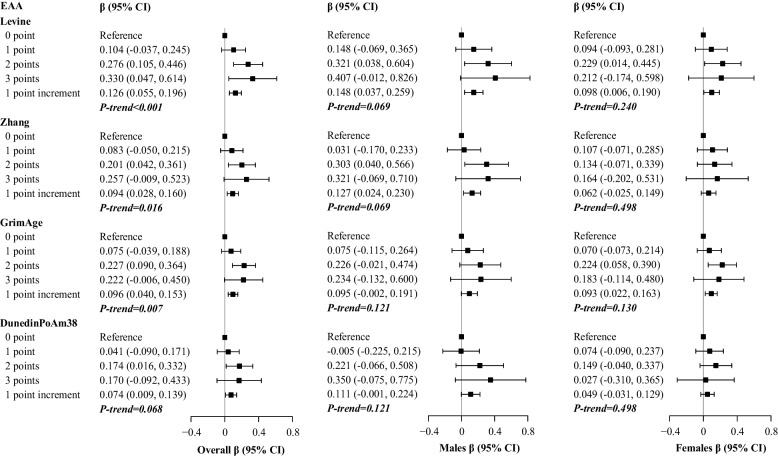
Also, a greater composite score of variability was associated with faster EAA (z-score of the regression residual of the epigenetic clock on chronological age) in four second-generation clocks trained on health outcomes, and the associations were consistently observed in both male and female participants (Fig. 3). Each point increment in the score was related to faster EAA by 0.126 z-score (95% CI: 0.055, 0.196) in the Levine clock, 0.094 z-score (95% CI: 0.028, 0.160) in the Zhang clock, 0.096 z-score (95% CI: 0.040, 0.153) in the GrimAge clock, and 0.074 z-score (95% CI: 0.009, 0.139) in DunedinPoAm38. All tests were significant after B-H adjustments (adjusted P-values < 0.05) except for that of DunedinPoAm38 (adjusted P-value = 0.068). The relations were non-significant in most first-generation clocks that are trained on chronological age, although the direction was similar. For example, the standardized coefficient (95% CI) was 0.051 (− 0.020, 0.122) for the Horvath 1 clock. Details were displayed in Additional file 1: Table S3.
Fig. 3.
Multivariable adjusted differences and 95% confidence intervals (CIs) in epigenetic age acceleration indicated by second-generation clocks associated with the composite score of variability in the overall population and by gender. EAA, epigenetic age acceleration. β-Coefficients were derived from models adjusted for age (continuous), gender (female or male, unless in gender-stratified analyses), education (high school degree level, yes/no), marriage status (yes/no), race (White/Black/others), drinking status (current/ever/never), smoking status (current/ever/never), physical activity (> 1 time per week/1–3 times per month/never), household income (in quartiles), body mass index, systolic blood pressure, and pulse rate (all continuous) in 2014/2016. P-values were adjusted using the Benjamini-Hochberg methods
Associations of BWV, PRV, and BPV with mortality and epigenetic age acceleration
We observed non-heterogeneous associations of BWV, PRV, and BPV with mortality in both HRS and CHARLS (P-heterogeneity > 0.05, Table 2). The pooled HRs (95% CIs) of mortality for each SD increment in BWV, PRV, and BPV were 1.21 (95% CI: 1.15, 1.28), 1.06 (1.00, 1.13), and 1.12 (1.04, 1.19), respectively. The corresponding HRs (95% CIs) were 1.24 (1.15, 1.33), 1.07 (0.99, 1.16), 1.11 (1.03, 1.21) in the HRS and 1.17 (1.07, 1.29), 1.05 (0.95, 1.17), 1.12 (1.01, 1.24) in the CHARLS.
Table 2.
Multivariable adjusted hazard ratios (HRs)a and 95% confidence intervals (CIs) of mortality associated with body weight variability, pulse rate variability, and blood pressure variability in the HRS and CHARLS
| Variable | HRS | CHARLS | Pooled HRb | P-heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|
| Median, % | Cases/person-years | HR (95% CI) | Median, % | Cases/person-years | HR (95% CI) | |||
| Body weight variability | ||||||||
| Tertile 1 | 1.9 | 124/6934 | 1.00 (reference) | 1.5 | 79/6894 | 1.00 (reference) | 1.00 (reference) | / |
| Tertile 2 | 4.0 | 129/6900 | 1.01 (0.78, 1.30) | 3.1 | 85/6895 | 1.16 (0.85, 1.58) | 1.07 (0.88, 1.30) | 0.499 |
| Tertile 3 | 8.0 | 230/6662 | 1.67 (1.33, 2.11) | 6.0 | 131/6892 | 1.57 (1.18, 2.09) | 1.63 (1.36, 1.95) | 0.742 |
| Per SD increment | 1.24 (1.15, 1.33) | 1.17 (1.07, 1.29) | 1.21 (1.15, 1.28) | 0.336 | ||||
| P-value | < 0.001 | 0.001 | ||||||
| Pulse rate variability | ||||||||
| Tertile 1 | 3.9 | 148/6841 | 1.00 (reference) | 3.8 | 77/6898 | 1.00 (reference) | 1.00 (reference) | / |
| Tertile 2 | 8.0 | 134/6830 | 0.86 (0.68, 1.09) | 7.6 | 95/6894 | 1.07 (0.79, 1.45) | 0.93 (0.78, 1.13) | 0.265 |
| Tertile 3 | 14.3 | 201/6825 | 1.13 (0.91, 1.41) | 13.3 | 123/6889 | 1.17 (0.87, 1.57) | 1.14 (0.96, 1.36) | 0.853 |
| Per SD increment | 1.07 (0.99, 1.16) | 1.05 (0.95, 1.17) | 1.06 (1.00, 1.13) | 0.777 | ||||
| P-value | 0.132 | 0.341 | ||||||
| Blood pressure variability | ||||||||
| Tertile 1 | 4.2 | 131/6787 | 1.00 (reference) | 4.0 | 87/6894 | 1.00 (reference) | 1.00 (reference) | / |
| Tertile 2 | 8.2 | 140/6933 | 0.98 (0.77, 1.25) | 8.0 | 92/6889 | 0.93 (0.69, 1.25) | 0.96 (0.79, 1.16) | 0.789 |
| Tertile 3 | 14.4 | 212/6776 | 1.20 (0.96, 1.50) | 13.6 | 116/6898 | 1.14 (0.86, 1.51) | 1.17 (0.99, 1.40) | 0.780 |
| Per SD increment | 1.11 (1.03, 1.21) | 1.12 (1.01, 1.24) | 1.12 (1.04, 1.19) | 0.893 | ||||
| P-value | 0.073 | 0.036 | ||||||
HRS Health and Retirement Study, CHARLS China Health and Retirement Longitudinal Study, SD standard deviation
aHazard ratios were adjusted for age (continuous), gender (female/male), education (high school degree level, yes/no), marriage status (yes/no), residence (rural/urban) in the CHARLS, race (White/Black/others) in the HRS, drinking status (current/ever/never), smoking status (current/ever/never), physical activity (> 1 time per week/1–3 times per month/never in the HRS and any/never/missing in the CHARLS), household income (quartiles in the HRS and ≤ 9999 yuan/≥ 10000 yuan/missing in the CHARLS), and body mass index/systolic blood pressure/pulse rate (all continuous) in 2014/2016 (HRS) or in 2015 (CHARLS)
bHRs were pooled using fixed effect models
In the HRS-VBS, the associations of BWV, PRV, and BPV with EAA varied across epigenetic clocks (Additional file 1: Fig. S2 and Table S4). Larger PRV was related to significantly faster EAA in GrimAge (β for per SD increment = 0.055 z-score, 95% CI: 0.005, 0.104). Larger BPV was associated with significantly faster EAA in Zhang (0.079 z-score, 95% CI: 0.020, 0.138) and Horvath 1 (0.084 z-score, 95% CI: 0.020, 0.147). For other non-significant associations, the directions were generally consistent with our hypotheses.
Sensitivity analyses
The results in the sensitivity analyses were consistent with the primary findings (Additional file 1: Tables S5 and S6). When further adjusted for major health conditions, the relations of the composite score of variability to mortality and EAA persisted. When SD and VIM were used to measure the variability in physiological measures, the associations were comparable to the primary findings. When we replaced SBPV with DBPV, the relations were generally unchanged. When we redefined the cutoffs for large variability as medians or alternatively assigned 0, 1, and 2 to the increasing tertiles of variability indicators, the relations of the composite score to mortality and EAA remained similar. When further adjusted for cell-type proportions in the analysis of EAA, the relations were generally the same.
Discussion
In two independent large cohorts of middle-aged and older adults in the USA and China, a higher composite score of long-term variability in body weight, pulse rate, and blood pressure was related to a higher risk of mortality independent of their baseline levels. Also, a higher score of variability was associated with faster EAA in all four second-generation epigenetic clocks that are trained on health outcomes. Aggregately, our findings suggest that instability in major physiological measures might be an important predictor of mortality and an indicator of biological aging in middle-aged and older adults.
The current study is one of the few that has assessed the relations of variabilities in physiological measures to mortality. In previous studies, BWV [5], PRV [55], and BPV [16] have been associated with mortality separately. For example, in the Framingham Heart Study, the highest degree of BWV was related to a higher risk (27% in men and 65% in women) of mortality as compared with those with the lowest BWV, which was confirmed by our analysis [5]. Although the individual associations of the variabilities in physiological measures with health outcomes have been widely explored [5, 33, 55, 56], little is known about their combined association with health outcomes. In a cohort study of Korean adults, larger 13-year variability in blood pressure, glucose, cholesterol, and BMI were related to higher mortality risk in the subsequent 3 years, and the HR (95% CI) for mortality was 1.21 (1.18, 1.23) for each unit increment in the score (range: 0–4) combining these indicators [33]. In our study, the combined and individual associations of 8-year (in HRS) or 4-year (in CHARLS) variability in physiological measures with mortality were consistently strong in two nationally representative cohorts in the USA and China, and the associations were independent of the baseline levels of these physiological measures. Our findings suggested that a composite score constructed with long-term variability indicators in non-invasive measurements may be a novel predictor or risk factor for mortality. However, whether the composite score is associated with long-term risk of mortality and whether the associations are causal warrant further investigation.
To our knowledge, this is the first study relating long-term variability in physiological measures to epigenetic aging. Previous studies have suggested that physiological dysfunction might be a result of epigenomic modifications [40], but the association between them was far from conclusive [57]. Our findings added valuable evidence to the current literature that long-term variability in physiological measures could be predictors of EAA, which implied a potential link between physiological homeostasis and DNA methylation [58]. Moreover, we found that the relation was more significant in the second-generation clocks than the first-generation clocks, potentially because the second-generation clocks are more relevant to aging-related health outcomes than the first-generation clocks. Although the underlying biological pathways remain largely unknown, previous studies have suggested a role for methylation in the crosstalk between the adaptive immune system and physiological homeostasis [59]. Also, global DNA methylation might also reflect fat distribution and homeostasis of certain biomarkers, although the direction of the causal link has yet to be confirmed [59]. Overall, by incorporating EAA, a reliable estimation of biological aging [40], our findings highlight the ability of physiological variability to reflect the pace of biological aging. Our findings that the observed association was independent of baseline levels of physiological status may suggest that longitudinal dynamic physiological statuses are important additions to their static values in the assessment of the pace of aging.
Several possible pathways could explain the observed associations, including cardiac and vascular regulation and inflammation, which were related to mortality and biological aging, respectively. For example, BPV and PRV were associated with poor cardiac and vascular regulation [60, 61], and BWV was linked to adipose tissue inflammation [62] and adipokine secretion alteration [63, 64]. Furthermore, the disturbance of homeostasis is often accompanied by inflammation [65], which may be linked to DNA methylation and demethylation processes [66]. However, current evidence is not sufficient for the assertion of their causal relation, as vascular and metabolic dysfunction [67–70] could be the common reasons for the loss of homeostasis and faster aging. More experimental and observational studies are required to further reveal the complex relations and underlying mechanisms.
The strengths of the present study included the two nationally representative cohorts, the high rates of follow-up, the objectively measured exposures, and the relatively large sample sizes. The high-quality epigenetic clock data also enabled us to further assess the association from a novel perspective. Nevertheless, our findings should be interpreted with caution due to some limitations. First, our findings did not necessarily suggest a causal link between the variability indicators and mortality or EAA, as residual confounding might still exist. For example, we did not fully account for intentional physiological changes, and physiological instability due to underlying diseases could not be fully eliminated. Second, the follow-up duration for mortality was relatively short, and future studies are needed to investigate the association of variability in physiological measures with mortality over a longer term. Third, we only included participants who attended multiple physical examinations, which limited the generalizability of our findings as they might be healthier than the general population. Moreover, while the indicators were significantly related to EAA, the overall variation explained was relatively low in most clocks (< 2% in all clocks, data not shown). Although previous studies also reported a low variance explained when assessing some well-established risk factors of aging, this might reflect the measurement errors in DNA methylation. Lastly, the generalizability of our findings on EAA should be validated in other populations, especially given that DNA methylation may differ across races and regions [71].
Conclusions
Our prospective study suggests that a higher composite score of long-term variability in physiological measures, including body weight, pulse rate, and blood pressure, is related to an increased risk of mortality and faster epigenetic age acceleration independent of their baseline levels. Our findings underscored the potential role of homeostasis maintenance in the aging process. Future research is needed to confirm the study findings and to explore the underlying biological mechanisms.
Supplementary Information
Additional file 1: Fig. S1. Participant inclusion and exclusion flowchart. Fig. S2. Scatter plots of BWV, PRV, and BPV with epigenetic age acceleration indicated by 4 second-generation epigenetic clocks. Table S1. Descriptive statistics of the 13 epigenetic clocks (N=1,047). Table S2. Associations of the composite score of variability in physiological measures with mortality in the HRS and CHARLS with different adjustments. Table S3. Associations between each one-point increment in the composite score of variability and epigenetic age acceleration indicated by 13 first- and second-generation epigenetic clocks. Table S4. Associations of BWV, PRV and SBPV with epigenetic age acceleration indicated by 13 first- and second-generation epigenetic clocks. Table S5. Sensitivity analyses for the associations between each one-point increment in the composite score of variability with risk of all-cause mortality. Table S6. Sensitivity analyses for the associations between variability of physiological measures and epigenetic age acceleration.
Acknowledgements
The Health and Retirement Study is sponsored by the National Institute on Aging (NIA U01AG009740) and the Social Security Administration. The study director is Dr. David R. Weir of the Survey Research Center at the University of Michigan’s Institute for Social Research. The China Health and Retirement Longitudinal Study was funded by the National Institute on Aging (R01-AG037031 and R01AG067625), the Natural Science Foundation of China (72061137005, 81903392, 71603013, 72173008, and 71873010), and the China Medical Board (20-364). The authors would like to thank the participants and workers of the HRS and CHARLS for their selfless contributions which made the current study possible.
Abbreviations
- BMI
Body mass index
- BPV
Blood pressure variability
- BWV
Body weight variability
- CHARLS
China Health and Retirement Longitudinal Study
- CI
Confidence interval
- CV
Coefficient of variation
- DBPV
Diastolic blood pressure variability
- EAA
Epigenetic age acceleration
- HR
Hazard ratio
- HRS
Health and Retirement Study
- PRV
Pulse rate variability
- SBPV
Systolic blood pressure variability
- SD
Standard deviation
- VBS
Venous Blood Study
Authors’ contributions
C. Y. and H. C. designed the study. T. Z. performed the statistical analyses. H. C., T. Z., S. W., and Y. C. interpreted the data. H. C. and T. Z. drafted the manuscript. C. Y., S. W., Y. C., and G. Z. further revised the manuscript. C. Y. supervised the data analysis and interpretation. C.Y. had the primary responsibility for the study’s final content. All authors critically reviewed the manuscript and approved the final draft.
Funding
This work was supported by the Zhejiang University Global Partnership Fund (granted to CY) and the Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province (granted to YC, Grant No: 2020E10004). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and materials
The datasets that support the findings of this study are available on the HRS website (https://hrsonline.isr.umich.edu/) [23] and the CHARLS website (http://charls.pku.edu.cn/en/) [24].
Declarations
Ethics approval and consent to participate
The HRS and HRS-VBS were approved by the Health Sciences and Behavioral Sciences institutional review board at the University of Michigan (HUM00061128). The CHARLS was approved by the Ethics Committee of Pecking University (00001052-11, 014).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hui Chen and Tianjing Zhou contributed equally as co-first authors.
References
- 1.Feng Z, Glinskaya E, Chen H, Gong S, Qiu Y, Xu J, et al. Long-term care system for older adults in China: policy landscape, challenges, and future prospects. Lancet. 2020;396:1362–1372. doi: 10.1016/S0140-6736(20)32136-X. [DOI] [PubMed] [Google Scholar]
- 2.Bengtson V. Global aging and challenges to families. New York: Routledge; 2017. [Google Scholar]
- 3.Lee J, Phillips D, Wilkens J, Chien S, Lin Y-C, Angrisani M, et al. Cross-country comparisons of disability and morbidity: evidence from the gateway to global aging data. J Gerontol A Biol Sci Med Sci. 2018;73:1519–1524. doi: 10.1093/gerona/glx224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harman D. Aging: overview. Ann N Y Acad Sci. 2001;928:1–21. doi: 10.1111/j.1749-6632.2001.tb05631.x. [DOI] [PubMed] [Google Scholar]
- 5.Lissner L, Odell PM, D’Agostino RB, Stokes J, Kreger BE, Belanger AJ, et al. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324:1839–1844. doi: 10.1056/NEJM199106273242602. [DOI] [PubMed] [Google Scholar]
- 6.Chen TT, Maevsky EI, Uchitel ML. Maintenance of homeostasis in the aging hypothalamus: the central and peripheral roles of succinate. Front Endocrinol (Lausanne) 2015;6:7. doi: 10.3389/fendo.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med. 2015;163:329–338. doi: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeBlanc ES, Rizzo JH, Pedula KL, Yaffe K, Ensrud KE, Cauley J, et al. Weight trajectory over 20 years and likelihood of mild cognitive impairment or dementia among older women. J Am Geriatr Soc. 2017;65:511–519. doi: 10.1111/jgs.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho H-E, Yeh C-J, Chu W-M, Lee M-C. Midlife body mass index trajectory and risk of frailty 8 years later in Taiwan. J Nutr Health Aging. 2019;23:849–855. doi: 10.1007/s12603-019-1226-6. [DOI] [PubMed] [Google Scholar]
- 10.Anker D, Santos-Eggimann B, Zwahlen M, Santschi V, Rodondi N, Wolfson C, et al. Blood pressure in relation to frailty in older adults: a population-based study. J Clin Hypertens. 2019;21:1895–1904. doi: 10.1111/jch.13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dwivedi AK, Dubey P, Cistola DP, Reddy SY. Association between obesity and cardiovascular outcomes: updated evidence from meta-analysis studies. Curr Cardiol Rep. 2020;22:25. doi: 10.1007/s11886-020-1273-y. [DOI] [PubMed] [Google Scholar]
- 12.Fang S-C, Wu Y-L, Tsai P-S. Heart rate variability and risk of all-cause death and cardiovascular events in patients with cardiovascular disease: a meta-analysis of cohort studies. Biol Res Nurs. 2020;22:45–56. doi: 10.1177/1099800419877442. [DOI] [PubMed] [Google Scholar]
- 13.Hughes D, Judge C, Murphy R, Loughlin E, Costello M, Whiteley W, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA. 2020;323:1934–1944. doi: 10.1001/jama.2020.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CM, Woodward M, Batty GD, Beiser AS, Bell S, Berr C, et al. Association of anthropometry and weight change with risk of dementia and its major subtypes: a meta-analysis consisting 2.8 million adults with 57 294 cases of dementia. Obes Rev. 2020;21:e12989. doi: 10.1111/obr.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Y-C, Huang Y-C, Huang W-L. Heart rate variability in patients with dementia or neurocognitive disorders: a systematic review and meta-analysis. Aust N Z J Psychiatry. 2022;56:16–27. doi: 10.1177/0004867420976853. [DOI] [PubMed] [Google Scholar]
- 16.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. doi: 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19:371–384. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- 18.Ferrucci L, Gonzalez-Freire M, Fabbri E, Simonsick E, Tanaka T, Moore Z, et al. Measuring biological aging in humans: a quest. Aging Cell. 2020;19:e13080. doi: 10.1111/acel.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oblak L, van der Zaag J, Higgins-Chen AT, Levine ME, Boks MP. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res Rev. 2021;69:101348. doi: 10.1016/j.arr.2021.101348. [DOI] [PubMed] [Google Scholar]
- 21.Quach A, Levine ME, Tanaka T, Lu AT, Chen BH, Ferrucci L, et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging. 2017;9:419–446. doi: 10.18632/aging.101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crimmins EM, Thyagarajan B, Levine ME, Weir DR, Faul J. Associations of age, sex, race/ethnicity, and education with 13 epigenetic clocks in a nationally representative U.S. sample: the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2021;76:1117–1123. doi: 10.1093/gerona/glab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Health and Retirement Study. https://hrs.isr.umich.edu/about?_ga=2.82991632.761114232.1668737860-1578651410.1635161200. Accessed 18 Nov 2022.
- 24.China Health and Retirement Longitudinal Survey. http://charls.pku.edu.cn/en/. Accessed 18 Nov 2022.
- 25.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JWR, Weir DR. Cohort profile: the Health and Retirement Study (HRS) Int J Epidemiol. 2014;43:576–585. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS) Int J Epidemiol. 2014;43:61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crimmins E, Faul J, Thyagarajan B, Weir D. Venous blood collection and assay protocol in the 2016 Health and Retirement Study. 2016. [Google Scholar]
- 28.Crimmins E, Guyer H, Langa K, Ofstedal MB, Wallace R, Weir D. Documentation of physical measures, anthropometrics and blood pressure in the health and retirement study. Ann Arbor: Survey Research Center University of Michigan; 2008. [Google Scholar]
- 29.Cai X, Qiu S, Liu S, Lu Y, Luo D, Li R, et al. Body-weight fluctuation and risk of diabetes in older adults: the China Health and Retirement Longitudinal Study (CHARLS) Diabetes Res Clin Pract. 2020;169:108419. doi: 10.1016/j.diabres.2020.108419. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Meng Q, Yang C, Wang Y, Kong G, Zhao Y, et al. Association between pulse pressure, systolic blood pressure and the risk of rapid decline of kidney function among general population without hypertension: results from the China health and retirement longitudinal study (CHARLS) J Transl Med. 2021;19:512. doi: 10.1186/s12967-021-03176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Y, Wolters FJ, Chibnik LB, Licher S, Ikram MA, Hofman A, et al. Variation in blood pressure and long-term risk of dementia: a population-based cohort study. PLoS Med. 2019;16:e1002933. doi: 10.1371/journal.pmed.1002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153:598–615. doi: 10.1001/archinte.1993.00410050036006. [DOI] [PubMed] [Google Scholar]
- 33.Kim MK, Han K, Park Y-M, Kwon H-S, Kang G, Yoon K-H, et al. Associations of variability in blood pressure, glucose and cholesterol concentrations, and body mass index with mortality and cardiovascular outcomes in the general population. Circulation. 2018;138:2627–2637. doi: 10.1161/CIRCULATIONAHA.118.034978. [DOI] [PubMed] [Google Scholar]
- 34.Stephan Y, Sutin AR, Luchetti M, Terracciano A. Facets of conscientiousness and longevity: findings from the Health and Retirement Study. J Psychosom Res. 2019;116:1–5. doi: 10.1016/j.jpsychores.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang R, Liu Y, Wang H, Du Y. Leisure-time daily walking and blood pressure among Chinese older adults: evidence from the China Health and Retirement Longitudinal Study (CHARLS) Res Gerontol Nurs. 2019;12:248–258. doi: 10.3928/19404921-20190702-01. [DOI] [PubMed] [Google Scholar]
- 36.Crimmins E, Kim JK, Fisher J, Faul J. HRS epigenetic clocks. Ann Arbor: University of Michigan; 2020.
- 37.Schmitz LL, Zhao W, Ratliff SM, Goodwin J, Miao J, Lu Q, et al. The socioeconomic gradient in epigenetic ageing clocks: evidence from the multi-ethnic study of atherosclerosis and the Health and Retirement Study. Epigenetics. 2022;17(6):589–611. [DOI] [PMC free article] [PubMed]
- 38.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10:573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horvath S, Oshima J, Martin GM, Lu AT, Quach A, Cohen H, et al. Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria syndrome and ex vivo studies. Aging (Albany NY) 2018;10:1758–1775. doi: 10.18632/aging.101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin Q, Wagner W. Epigenetic aging signatures are coherently modified in cancer. PLoS Genet. 2015;11:e1005334. doi: 10.1371/journal.pgen.1005334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weidner CI, Lin Q, Koch CM, Eisele L, Beier F, Ziegler P, et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 2014;15:R24. doi: 10.1186/gb-2014-15-2-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vidal-Bralo L, Lopez-Golan Y, Gonzalez A. Simplified assay for epigenetic age estimation in whole blood of adults. Front Genet. 2016;7:126. doi: 10.3389/fgene.2016.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 2019;11:303–327. doi: 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Z, Wong A, Kuh D, Paul DS, Rakyan VK, Leslie RD, et al. Correlation of an epigenetic mitotic clock with cancer risk. Genome Biol. 2016;17:205. doi: 10.1186/s13059-016-1064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Wilson R, Heiss J, Breitling LP, Saum K-U, Schöttker B, et al. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat Commun. 2017;8:14617. doi: 10.1038/ncomms14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bocklandt S, Lin W, Sehl ME, Sánchez FJ, Sinsheimer JS, Horvath S, et al. Epigenetic predictor of age. PLoS One. 2011;6:e14821. doi: 10.1371/journal.pone.0014821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garagnani P, Bacalini MG, Pirazzini C, Gori D, Giuliani C, Mari D, et al. Methylation of ELOVL2 gene as a new epigenetic marker of age. Aging Cell. 2012;11:1132–1134. doi: 10.1111/acel.12005. [DOI] [PubMed] [Google Scholar]
- 50.Belsky DW, Caspi A, Arseneault L, Baccarelli A, Corcoran DL, Gao X, et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife. 2020;9:e54870. doi: 10.7554/eLife.54870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guevara EE, Lawler RR. Epigenetic clocks. Evol Anthropol. 2018;27:256–260. doi: 10.1002/evan.21745. [DOI] [PubMed] [Google Scholar]
- 52.Savin NE. Multiple hypothesis testing. Handb Econ. 1984;2:827–879. [Google Scholar]
- 53.Adalsteinsson BT, Gudnason H, Aspelund T, Harris TB, Launer LJ, Eiriksdottir G, et al. Heterogeneity in white blood cells has potential to confound DNA methylation measurements. PLoS One. 2012;7:e46705. doi: 10.1371/journal.pone.0046705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jansen RJ, Tong L, Argos M, Jasmine F, Rakibuz-Zaman M, Sarwar G, et al. The effect of age on DNA methylation in whole blood among Bangladeshi men and women. BMC Genomics. 2019;20:704. doi: 10.1186/s12864-019-6039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X, Hidru TH, Han X, Zhang X, Liu Y, Wang B, et al. Link between elevated long-term resting heart rate variability and pulse pressure variability for all-cause mortality. J Am Heart Assoc. 2020;9:e014122. doi: 10.1161/JAHA.119.014122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park MJ, Choi KM. Association between variability of metabolic risk factors and cardiometabolic outcomes. Diabetes Metab J. 2022;46:49–62. doi: 10.4093/dmj.2021.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahmoud AM, Ali MM. Methyl donor micronutrients that modify DNA methylation and cancer outcome. Nutrients. 2019;11:E608. doi: 10.3390/nu11030608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kubben N, Misteli T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat Rev Mol Cell Biol. 2017;18:595–609. doi: 10.1038/nrm.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keller M, Kralisch S, Rohde K, Schleinitz D, Dietrich A, Schön MR, et al. Global DNA methylation levels in human adipose tissue are related to fat distribution and glucose homeostasis. Diabetologia. 2014;57:2374–2383. doi: 10.1007/s00125-014-3356-z. [DOI] [PubMed] [Google Scholar]
- 60.Lee S-H, Kim MK, Rhee E-J. Effects of cardiovascular risk factor variability on health outcomes. Endocrinol Metab (Seoul) 2020;35:217–226. doi: 10.3803/EnM.2020.35.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basson MD, Klug MG, Hostetter JE, Wynne J. Visit-to-visit variability of blood pressure is associated with hospitalization and mortality in an unselected adult population. Am J Hypertens. 2018;31:1113–1119. doi: 10.1093/ajh/hpy088. [DOI] [PubMed] [Google Scholar]
- 62.Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes. 2013;62:3180–3188. doi: 10.2337/db12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oh TJ, Moon JH, Choi SH, Lim S, Park KS, Cho NH, et al. Body-weight fluctuation and incident diabetes mellitus, cardiovascular disease, and mortality: a 16-year prospective cohort study. J Clin Endocrinol Metab. 2019;104:639–646. doi: 10.1210/jc.2018-01239. [DOI] [PubMed] [Google Scholar]
- 64.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 65.Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wielscher M, Mandaviya PR, Kuehnel B, Joehanes R, Mustafa R, Robinson O, et al. DNA methylation signature of chronic low-grade inflammation and its role in cardio-respiratory diseases. Nat Commun. 2022;13:2408. doi: 10.1038/s41467-022-29792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang Y-Z, Jiménez JM, Ou K, McCormick ME, Zhang L-D, Davies PF. Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-like factor 4 promoter in vitro and in vivo. Circ Res. 2014;115:32–43. doi: 10.1161/CIRCRESAHA.115.303883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao J, Goldberg J, Bremner JD, Vaccarino V. Global DNA methylation is associated with insulin resistance: a monozygotic twin study. Diabetes. 2012;61:542–546. doi: 10.2337/db11-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X, Li J, Zheng S, Luo Q, Zhou C, Wang C. Fasting insulin, insulin resistance, and risk of cardiovascular or all-cause mortality in non-diabetic adults: a meta-analysis. Biosci Rep. 2017;37:BSR20170947. doi: 10.1042/BSR20170947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang FF, Cardarelli R, Carroll J, Fulda KG, Kaur M, Gonzalez K, et al. Significant differences in global genomic DNA methylation by gender and race/ethnicity in peripheral blood. Epigenetics. 2011;6:623–629. doi: 10.4161/epi.6.5.15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Participant inclusion and exclusion flowchart. Fig. S2. Scatter plots of BWV, PRV, and BPV with epigenetic age acceleration indicated by 4 second-generation epigenetic clocks. Table S1. Descriptive statistics of the 13 epigenetic clocks (N=1,047). Table S2. Associations of the composite score of variability in physiological measures with mortality in the HRS and CHARLS with different adjustments. Table S3. Associations between each one-point increment in the composite score of variability and epigenetic age acceleration indicated by 13 first- and second-generation epigenetic clocks. Table S4. Associations of BWV, PRV and SBPV with epigenetic age acceleration indicated by 13 first- and second-generation epigenetic clocks. Table S5. Sensitivity analyses for the associations between each one-point increment in the composite score of variability with risk of all-cause mortality. Table S6. Sensitivity analyses for the associations between variability of physiological measures and epigenetic age acceleration.
Data Availability Statement
The datasets that support the findings of this study are available on the HRS website (https://hrsonline.isr.umich.edu/) [23] and the CHARLS website (http://charls.pku.edu.cn/en/) [24].