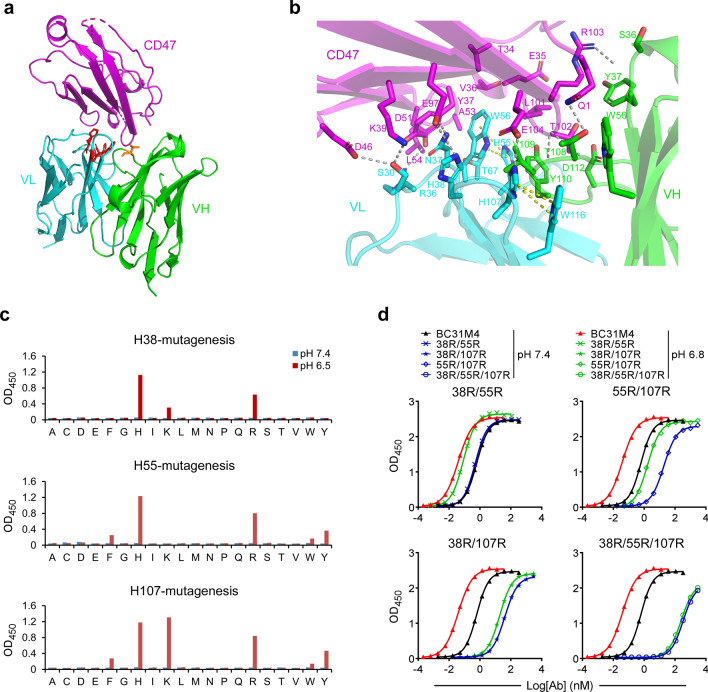
Fig. 2.
Structural characterization of the pH-dependent binding of BC31M4. a Crystal structure of the BC31M5-CD47 complex, depicted as ribbons. T108 (orange) in VH and histidines (red) in VL are shown as sticks. b Detailed view of the BC31M5-CD47 interface. Gray dashed lines indicate electrostatic interactions between BC31M5 and CD47. Side chains of contacted residues are shown as sticks. Yellow dashed lines indicate π-contacts. c The three histidines (H38, H55, or H107) in the VL of BC31M4 (in phage-scFv form) were mutated into any other amino acids individually (site saturation mutagenesis). These mutants binding to CD47 at pH7.4 and pH 6.5 were measured by ELISA. d Serial dilutions of BC31M4 mutants (in hIgG1 form) with the indicated double or triple histidine-to-arginine substitutions binding to CD47-ECD at pH7.4 and pH 6.8, measured by ELISA