Fig. 4: Deficiency of asc extends lifespan and abrogates pro-inflammatory il-1b upregulation in dpp9−/− zebrafish.
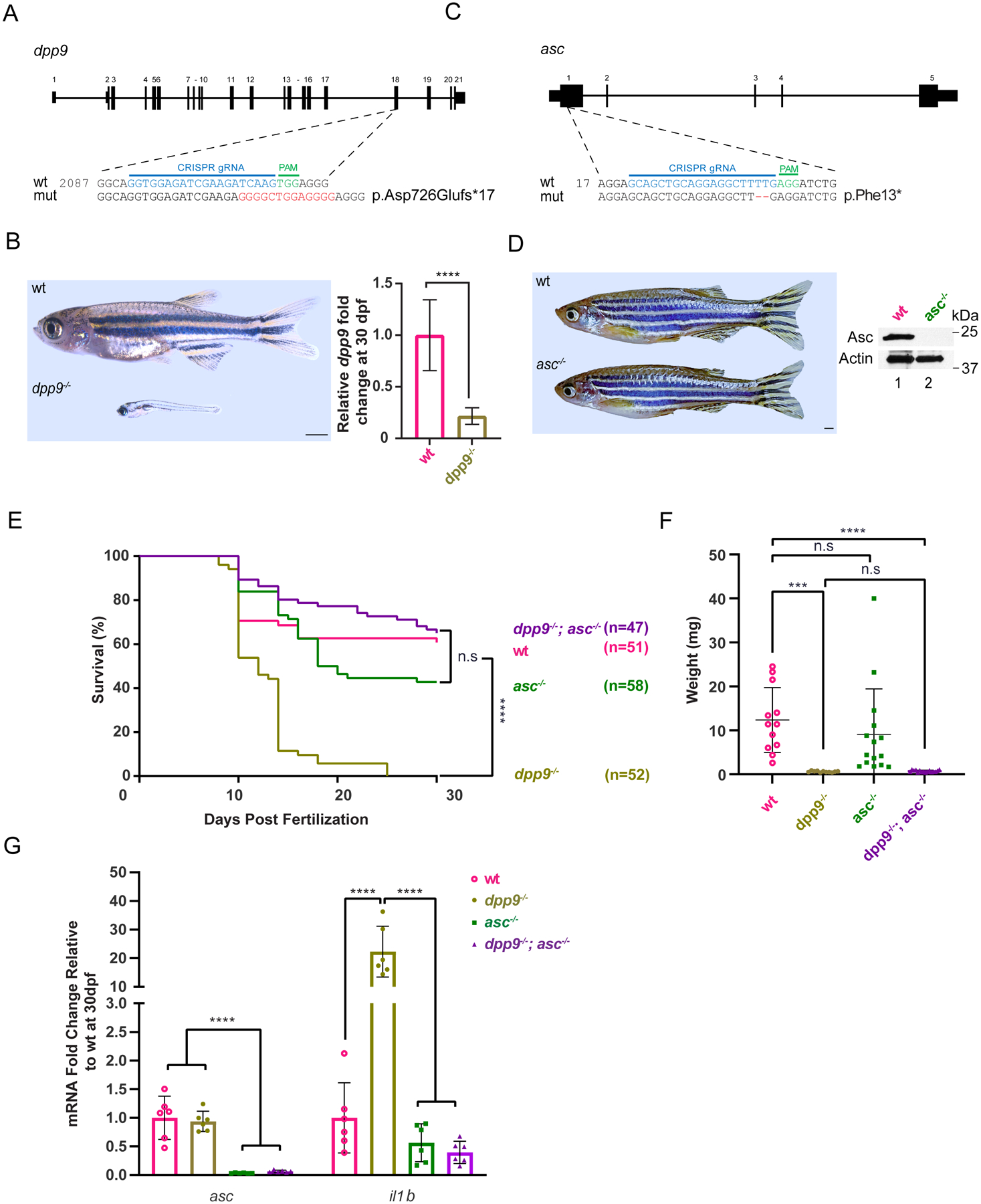
(A) Generation of dpp9 knockout zebrafish using CRISPR/Cas9-genome editing that resulted in an out-of-frame allele with premature stop codon. (B) Left, dpp9−/− zebrafish at 1 month of age exhibit a dramatic reduction in size (quantification shown in F). Right, validation of dpp9-KO by RT-qPCR. Data are mean ± SEM (n = 6). ****p<0.001 (one-way ANOVA).
(C) Generation of asc knockout zebrafish using CRISPR/Cas9 genome editing that resulted in a 2-bp deletion in exon 1 followed by a premature stop codon.
(D) Left, asc−/− zebrafish appeared indistinguishable from wt. Right, validation of complete asc-KO by western blotting.
(E) Kaplan-Meier survival curves of wt, dpp9−/−, asc−/− and double dpp9−/−; asc−/− zebrafish. Dpp9−/− zebrafish demonstrated a significant survival disadvantage that is rescued by inactivating asc. ****p<0.0001 (Logrank Mantel-Cox test).
(F) The weight of double dpp9−/−; asc−/− zebrafish is not rescued compared to that of dpp9−/− zebrafish. Data are mean ± SEM. ****p<0.001; ***p<0.01; n.s., non-significant (one-way ANOVA).
(G) In double dpp9−/−; asc−/− zebrafish, il1b expression is rescued to that of wt levels as demonstrated by RT-qPCR analysis. Data are mean ± SEM (n = 6). ****p<0.001 (one-way ANOVA).
