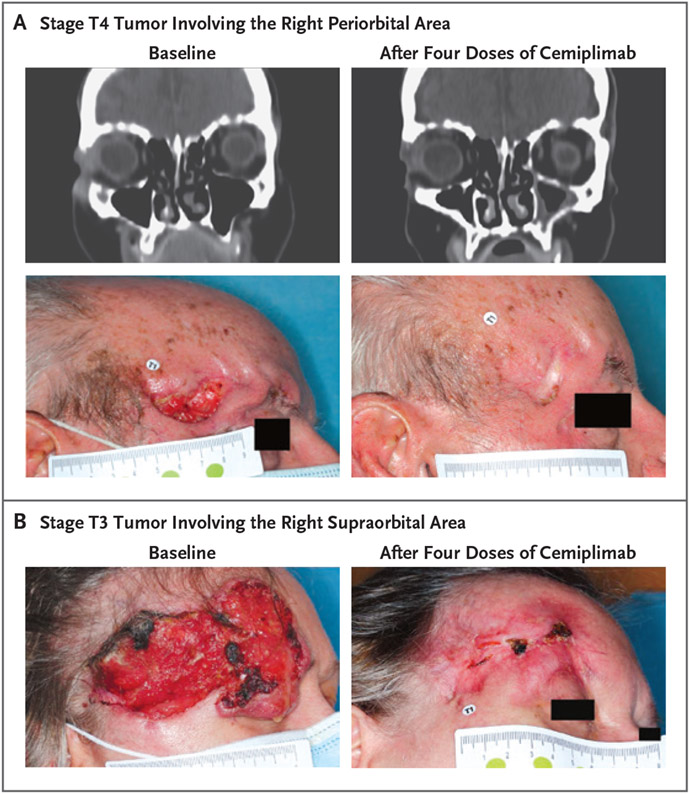
Figure 2. Clinical Responses in Patients with a Pathological Complete Response to Neoadjuvant Cemiplimab.
Panels A and B show photographs and contrast-enhanced computed tomographic (CT) images obtained from representative patients at baseline and after treatment with four doses of neoadjuvant cemiplimab, administered at a dose of 350 mg every 3 weeks. In Panel A, the patient is an 86-year-old man who presented with stage T4 cutaneous squamous-cell carcinoma involving the right periorbital area. In Panel B, the patient is a 59-year-old woman who presented with stage T3 cutaneous squamous-cell carcinoma involving the right supraorbital area. Neither patient underwent orbital exenteration, because the patients had a partial response on imaging, defined according to RECIST 1.1 and determined on investigator assessment, which allowed for less-extensive surgery.