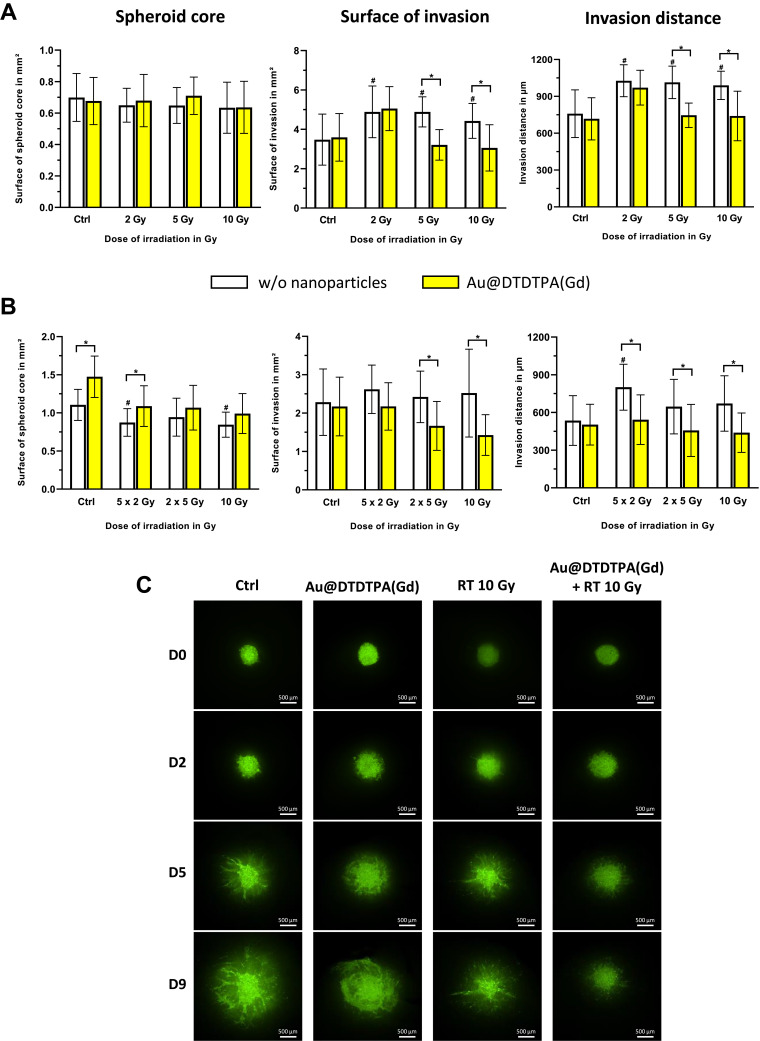
Figure 2.
In vitro anti-invasive effects of Au@DTDTPA(Gd) on irradiated cells. After seeding in 6-well plates (15 spheroids per well), U87 spheroids were exposed 5 mM Au@DTDTPA(Gd) for 24 h and/or treated by X-photon irradiation (w/o = without nanoparticles). (A) Influence of the dose of radiation. Single-dose of radiotherapy (2, 5 or 10 Gy) was delivered on T8 and each spheroid was then placed into individual well of 96-well plate and then embedded with 100 µL of a mix of Matrigel® and hyaluronic acid (100 µg/mL). (B) Influence of the radiation fractionation. Irradiated spheroids received either 10 Gy-monodose, 2 fractions of 5 Gy (2 X 5 Gy) or 5 fractions of 2 Gy (5 X 2 Gy) between T8 and T12 and spheroids were embedded in matrix at T13. Four days later, invasion of viable cells was imaged using the GelCount® system (Oxford Optronix, UK). Histograms represent for each treatment condition the measurements of the surface of spheroid core (mm²), the invasion surface (mm²) and the invasion distance (µm). They were measured with ImageJ software. Results are presented as mean ± SD (n ≥ 12 spheroids for at least n ≥ 3 independent experiments). # = significant difference between RT groups versus Ctrl group and * = significant difference between “with Au@DTDTPA(Gd) nanoparticles” groups versus “w/o nanoparticles” groups at p < 0.05 according to the Mann–Whitney U-test. (C) Invasion after treatments have also been addressed using organotypic brain slice cultures, that allowed untreated or treated U87-GFP spheroids to grow in vitro into cerebral environment. Representative images acquired at Day 0 (D0), Day 2 (D2), Day 5 (D5), Day 9 (D9) using a Nikon AZ100 fluorescence microscope (Nikon, France) show the growth of the tumor core and the progression of invasive area. The scale bar is 500 µm (n ≥ 3 independent experiments).