Figure 5. Chiral GC-MS analysis of modified PedA15 peptides.
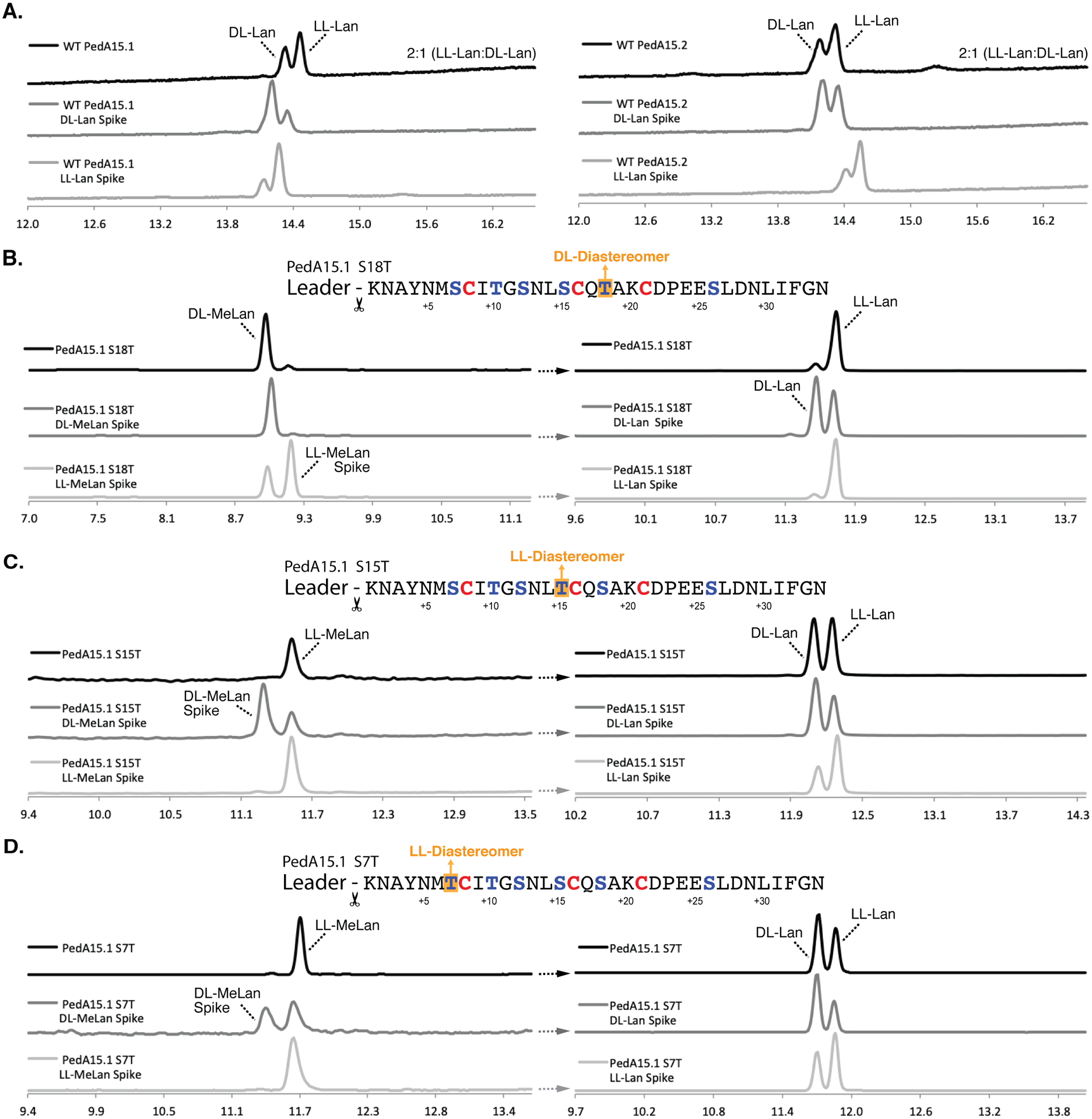
(A) Chiral GC-MS analysis of Ped15B/C-modified PedA15.1 (left) and PedA15.2 (right) core peptide fragments. See Materials and Methods for derivatization of the amino acids. Relative peak integration ratios for LL- and DL-Lan isomers are shown for each trace. (B) Ser-to-Thr mutation at position +18 from the GlyGly site resulted in the production of the DL-MeLan diastereomer (left). Lan in this mutant displayed solely the LL-Lan configuration (right). (C) Ser-to-Thr mutation at position +15 resulted in the production of the LL-MeLan diastereomer (left). Analysis of Lan in this sample revealed an approximately 1:1 ratio of DL- to LL-Lan (right). (D) Ser-to-Thr mutation at position +7 also resulted in the production of the LL-MeLan diastereomer (left). Analysis of Lan revealed an approximately 1:1 ratio of DL- to LL-Lan (right). For each set of traces, derivatized samples are shown in black and samples spiked with synthetic standards (DL- or LL-(Me)Lan, as indicated) are shown below in dark grey and light grey, respectively. Traces are selected-ion monitoring chromatograms for m/z = 365 (Lan) or 379 (MeLan), which correspond to the characteristic mass fragment for each derivatized compound. Retention times drift slightly and therefore coinjections with authentic standards were used to verify assignments.
