FIGURE 4.
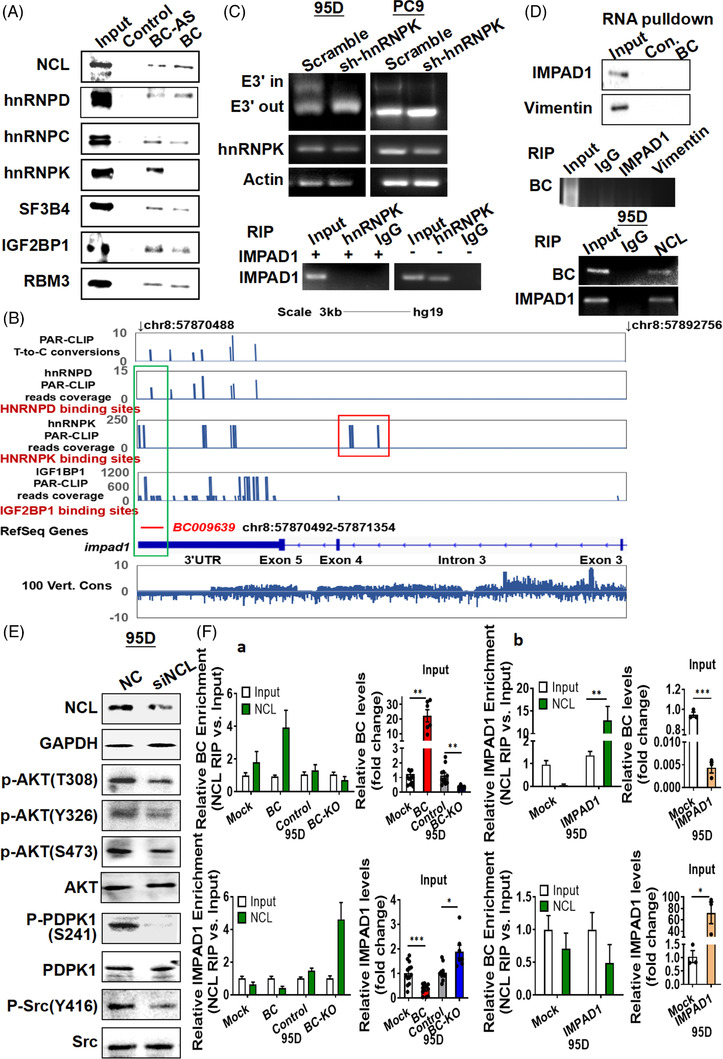
BC regulates inositol monophosphatase domain containing 1 (IMPAD1) alternative splicing via an interaction with hnRNPK and NCL. (A) Binding of BC to NCL, heterogeneous nuclear ribonucleoprotein (hnRNP), IGF2BP1, SF3B4 and RBM3 was analysed by BC RNA pull‐down assay. (B) Genome browser views of hnRNPD, hnRNPK and IGF2BP2 PAR‐CLIP binding sites and sequence conservation across vertebrates in genomic regions spanning IMPAD1 and BC. The aligned PAR‐CLIP reads are highlighted in BC‐aligned regions and IMPAD1 intron 3 with frame. (C) Splicing of IMPAD1‐203 exon 3′ was detected by PCR in 95D and PC9 cells following hnRNPK knock‐down (upper). RNA immunoprecipitation using the hnRNPK antibody was performed in 95D cells with IMPAD1 overexpression (lower). IgG‐bound RNA was used as a negative control. (D) IMPAD1 or vimentin binding to BC was analysed by BC RNA pull‐down and RNA immunoprecipitation (top, middle). Binding of NCL to BC and IMPAD1 mRNA were detected by RNA immunoprecipitation (bottom). (E) NCL, PDPK1, Src, AKT and their phosphorylation were detected by Western analysis in 95D cells with NCL knock‐down. (F) RNA immunoprecipitation and RT‐qPCR analysis were performed to measure BC and IMPAD1 mRNA binding to NCL protein in 95D cells with BC overexpression or knock‐down (a) or in 95D cells with IMPAD1 overexpression (b). Data are represented as mean ± SEM of at least three independent experiments. *p < .05, **p < .01
