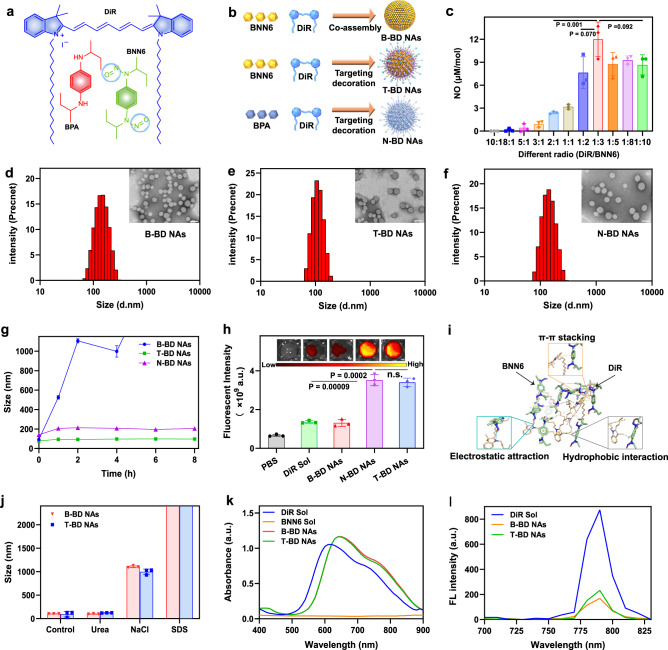
Fig. 2. Elaborate engineering and characterization of fuel pair nano-penetrator.
a Chemical structure of DiR, BNN6 (NO donor), and BPA (BNN6 precursor without NO production ability). b Co-assembly process of the binary NAs. c NO generation from B-BD NAs at various molar ratios of DiR and BNN6 from 10:1 to 1:10. Data are presented as mean ± SD (n = 3, independent experiments). Source data are provided as a Source Data file. One-way ANOVA (one-sided) with Dunnett’s multiple comparisons test was used for the analysis of data and adjusted P value. d–f Particle sizes distribution profiles and TEM images of B-BD NAs, T-BD NAs, and N-BD NAs, respectively, (Scale bar = 100 nm). Experiment was repeated three times independently with similar results. g Colloidal stability of B-BD NAs, T-BD NAs, and N-BD NAs incubated in PBS (pH 7.4) within 8 h at 37 ° C. Data are presented as mean ± SD (n = 3, independent experiments). h In vitro thrombus-targeting fluorescence intensity in artificial blood clots treated with PBS (pH 7.4), DiR Sol, B-BD NAs, N-BD NAs, and T-BD NAs, respectively. Data are presented as mean ± SD (n = 3, independent experiments). One-way ANOVA (one-sided) with Dunnett’s multiple comparisons test was used for the analysis of data and adjusted P value. The n.s. represent no significance. i Molecular docking simulation results of BNN6 and DiR. j Particle size changes of B-BD NAs and T-BD NAs in the presence of urea (50 mM), NaCl (50 mM), and SDS (50 mM), respectively (n = 3, independent experiments). k UV absorption spectra of DiR Sol, BNN6 Sol, B-BD NAs, and T-BD NAs. l Fluorescence spectra of DiR Sol, B-BD NAs, and T-BD NAs.