Figure 1.
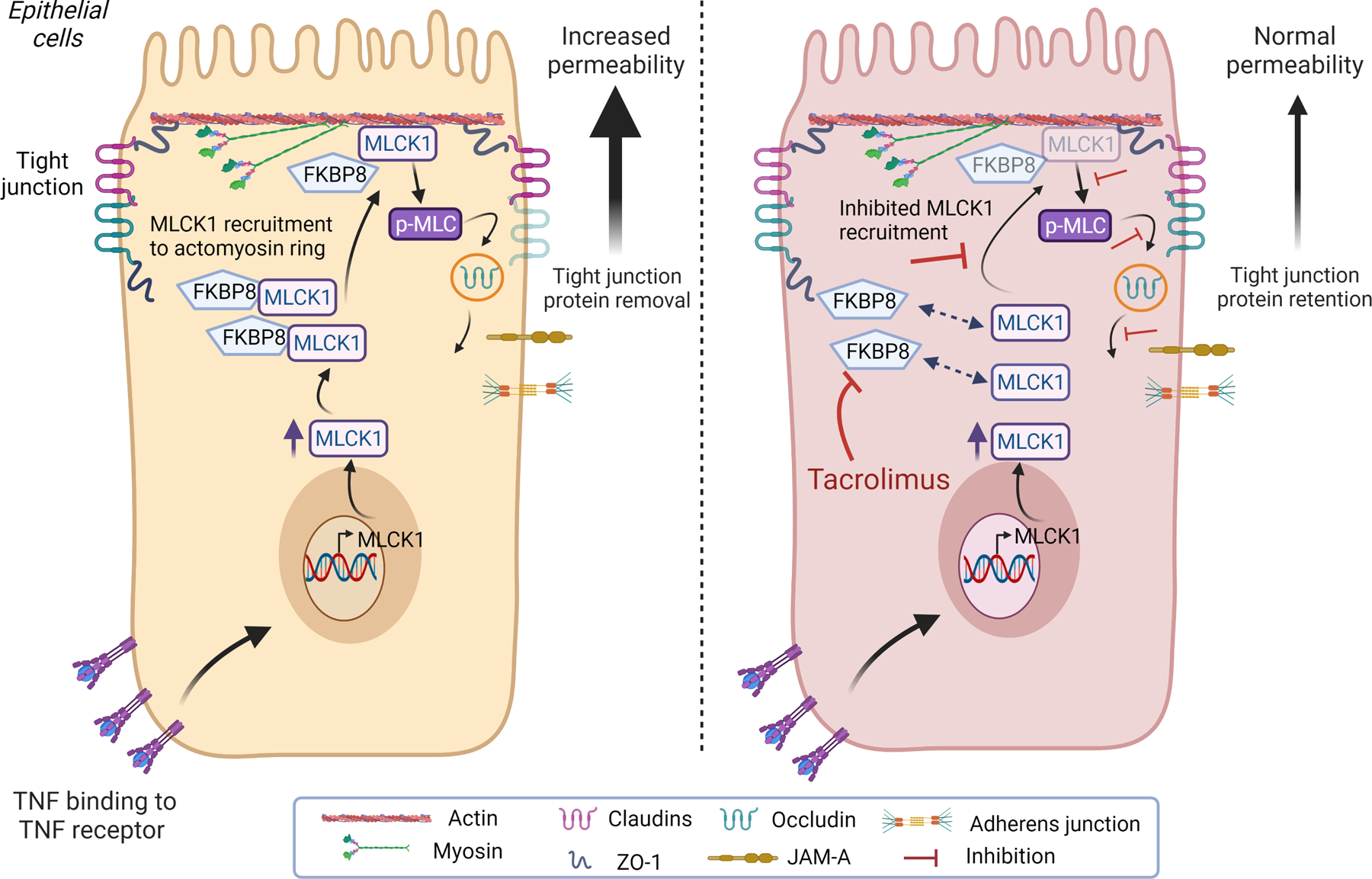
FKBP8 binding to MLCK1 mediates TNF-induced tight junction protein internalisation and increased permeability. TNF-α induces MLCK1 expression in intestinal epithelial cells. MLCK1 requires binding to the tacrolimus-sensitive protein FKBP8 in order to be recruited to the perijunctional actomyosin ring. MLCK1 can then induce phosphorylation of myosin light chain (pMLC), which drives internalisation of the transmembrane tight junction protein occludin. Removal of occludin from the tight junction increases paracellular permeability. Treatment of intestinal epithelial cells with tacrolimus has no effect on TNF induction of MLCK1 expression, but it causes dissociation of FKBP8 from MLCK1 thereby inhibiting MLCK1 recruitment to the perijunctional actomyosin ring. This inhibits MLCK1-mediated p-MLC, occludin internalisation and TNF-induced permeability increases. (figure created with BioRender.com). MLCK1, myosin light-chain kinase 1; TNF, tumour necrosis factor.
