Abstract
Introduction
In recent years, the erector spinae plane block (ESPB) has seen widespread use to treat acute and chronic pain in the regions of the thoracic spine. While limited data suggest its increasing utilization for pain management distal to the thoracic, abdomen and trunk, the anesthetic spread and analgesic mechanism of ESPB at the level of the lumbar spine has not been fully described or understood.
Methods
This is an observational anatomic cadaveric study to assess the distribution of solution following an ESPB block performed at the fourth lumbar vertebrae (L4) using ultrasound guidance to evaluate the spread of a 20 ml solution consisting of local anesthetic and methylene blue. The study was performed in an anatomy lab in a large academic medical center. Following injection of local anesthetic with contrast dye, cadaveric dissection was performed to better understand the extent of contrast dye and to determine the degree of staining to further predict analgesic potential. We reviewed the findings of other ESPB cadaveric studies currently available for comparison.
Results
Following cadaveric dissection in an anatomy lab, the contrast dye was observed in the ESP space, and staining was found most cranially at L2 and extending caudally underneath the sacrum. Evaluating the depth of its spread, we found it to be confined to the posterior compartment of the spine sparing the nerve roots bilaterally, which is consistent with the only other cadaveric study of ESPB performed at L4.
Conclusion
Our results demonstrate the clinical utility of lumbar ESPB where posterior confinement of local anesthesia is preferred. However, further investigation is needed to determine the efficacy of ESPB in lower extremity analgesia which is predicated on ventral nerve root involvement.
Keywords: Erector spinae block, Cadaver study, Regional anesthesia, Acute pain
Key Summary Points
| The anesthetic spread and analgesic mechanism of ESPB at the level of the lumbar spine have not been fully described or understood. |
| Different solutions and mixtures have been used in various studies. In our assessment we used 20 ml of solution to resemble a clinical setting. This should in theory provide a reasonable assessment of the spread of anesthetic in a fresh cadaver to estimate analgesic boundaries. |
| Evaluating the depth of its spread, we found it to be confined to the posterior compartment of the spine sparing the nerve roots bilaterally, which is consistent with the only other cadaveric study of ESPB performed at L4. |
Introduction
Postoperative pain control following lumbar spinal fusion can be very difficult and may lead to various complications. The pathophysiology of pain after spine surgery is multifactorial, involving nociceptive, inflammatory, and neuropathic pathways [1]. Various structures can be involved, including vertebral bodies, facet joints, intervertebral disks, muscle, fascia, ligaments, intra- and extraspinal nerves, dura, and subcutaneous/cutaneous tissues [2]. In an effort to reduce postoperative opiate requirement, new techniques in regional anesthesia have been developed as an adjunct to standard perioperative pain control. The erector spinae plane block (ESPB) has become an essential tool for the treatment of postoperative spinal pain, among other areas of the body from thoracic to truncal analgesia.
The ESPB was first described in 2016 as a new therapy for patients with severe thoracic neuropathic pain after bony injury and acute postoperative pain after video-assisted thoracoscopic wedge resection [3]. Since then there have been a number of reports of its effective use in managing postoperative pain for a variety of surgeries such as thoracic and cardiac surgery, breast surgery and reconstruction, bariatric surgery, laparoscopic ventral hernia repair and cholecystectomy, and various spinal procedures including scoliosis surgery and spinal decompression and fusion [4–13]. Previous literature has been retrospective to surmise efficacy or ESPB blocks have been performed at a more proximal mid to low thoracic level. The ESPB performed at lumbar spine brings a unique element of potential anterior spread of local anesthesia which can result in unwanted lower extremity weakness. Despite expanding use of this technique, the volume of literature of assessing the spread of injectate at lumbar region remains modest [4, 14–16]. At the time of this study, few cadaveric studies have been published to validate the anesthetic spread and analgesic mechanism of lumbar ESPBs but those that exist show conflicting evidence with varied volumes and mixtures of solution used and variety in the spread of said medications [17–26].
The use of lumbar ESPB for lumbar spinal surgeries, although promising, remains limited to a few case reports, a retrospective study, and one randomized controlled trial [9–13]. The purpose of this single cadaver dissection is to determine the extent of a 20 ml local anesthesia spread after the ESPB at lumbar region and enhance the depth of evidence currently available.
Study Objective
The objective of this observational anatomic study was to simulate the standard technique of an ultrasound-guided ESPB performed in a fresh cadaver at the transverse process of L4 performed in an academic cadaver lab setting. Following the nerve block, a layered dissection sought to evaluate the spread of a 20 ml solution consisting of local anesthetic and methylene blue. We assessed posteroanterior depth of penetration, flow away from midline, as well as craniocaudal spread to adjacent vertebral levels, particularly in relation to the dorsal and ventral rami of the lumbar spinal nerves with the hypothesis to provide clinical predictability for the analgesic road-mapping for perioperative analgesia of the spine.
Methods
The cadaver used in this observational study was provided for educational purposes by Avanos Medical, Inc. One fresh, un-embalmed cadaver was included and there was no history of spinal pathology or prior spine surgery reported. The cadaver was stored in the dissection area at room temperature for several hours prior to the start of the study.
Compliance with Ethics and Guidelines
Ethics committee approval was not required as this was a cadaveric study with the cadaver provided by Avanos Medical Inc. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Erector Spinae Plane Block Technique
Standard technique for the ESPB was performed by the lead investigator. With the cadaver in prone position, a Sonosite M-Turbo (Sonosite Inc., Bothell, WA, USA) ultrasound machine with a high-frequency linear transducer (13–6 MHz) was used to longitudinally scan and identify the sacrum and then moved cephalad to identify the spinous process of L4. The transducer was then moved laterally until the transverse process of L4 with overlying erector spinae muscle plane was identified. A 9 cm, 21 G needle (Arrow StimuQuik ECHO Stimulating and Echogenic Peripheral Nerve Block Needle) was inserted in-plane in a caudocranial direction until it was visualized contacting the dorsal surface of the transverse process of L4. A solution consisting of 1 ml of methylene blue and 19 ml of 0.25% bupivacaine was used to more closely approximate injectate used during ESPBs. The 20 ml solution was slowly injected, over approximately 30 s, under direct ultrasound visualization to confirm spread deep to the erector spinae muscle fascial plane (Fig. 1). The procedure was then repeated on the contralateral side.
Fig. 1.
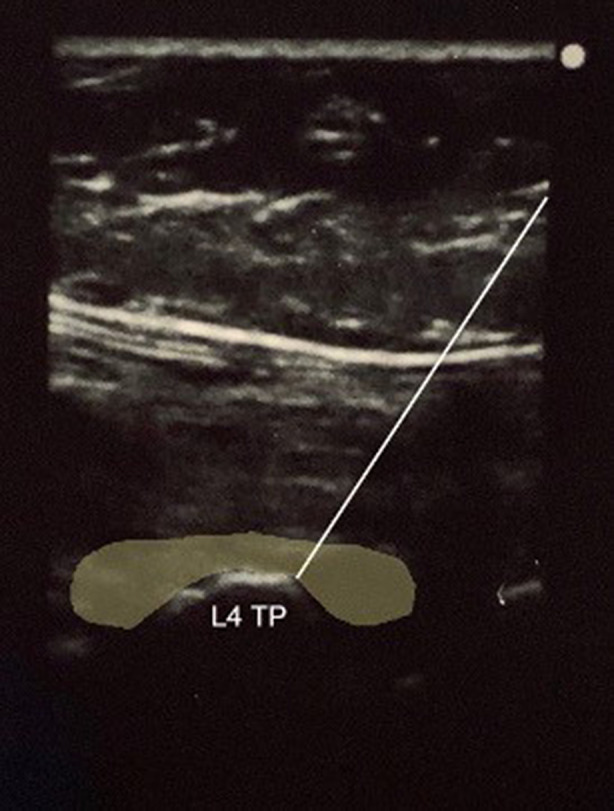
In-plane ultrasound-guided erector spinae plane block at the transverse process of L4 with highlighted area of injectate spread
Cadaveric Dissection
A vertical midline skin incision was made from the lower thoracic spine to the sacrum. This was followed by perpendicular incisions at T12 and S1 which extended laterally to the midpoint of the iliac crests; this approximated the mid scapular lines. An 11 blade scalpel was used to dissect down to the thoracodorsal fascia. This created a skin flap that, when reflected, improved visualization of deeper tissues while maintaining the integrity of the deeper muscular layers. After the thoracodorsal fascia was identified, the erector spinae and quadratus lumborum muscles were each vertically and horizontally incised and then reflected. This exposed the area of injectate spread, commensurate with needle introduction. The tissues deep to the erector spinae were blunt dissected to preserve the underlying neural and vascular tissue.
Results
A bilateral L4 ESPB was successfully performed at L4 in a single cadaver model. Appropriate craniocaudal injectate spread in the plane deep to the lumbar erector spinae muscles was observed using ultrasound (Fig. 1). After posteroanterior plane dissection, the injectate solution was observed to have spread in a craniocaudal direction consistently extending from L2 to the sacrum, bilaterally (Fig. 2). Staining from the methylene blue was found dorsal to the transverse processes at these levels and extending to the inside of the erector spinae muscles, covering the posterior rami. Dissection deep to the erector spinae showed that the dye did not reach the ventral rami of the spinal nerves (Fig. 3). There was no dye present in the subcutaneous layer other than trivial leakage that likely occurred during needle placement. No laminectomy was performed and the epidural space was not dissected.
Fig. 2.

Craniocaudal spread of injectate after bilateral ESPB performed at L4
Fig. 3.
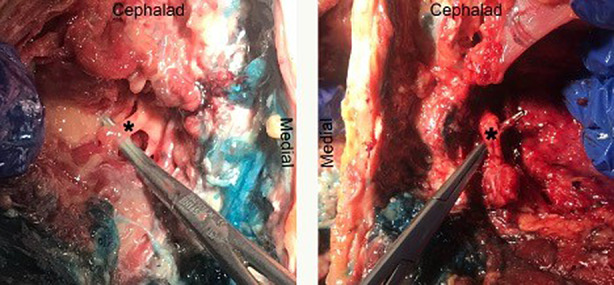
Dissection of ventral rami of L4 spinal nerve showing lack of injectate staining (left: left L4 ventral ramus; right: right L4 ventral ramus)
Discussion
The majority of ESPB studies, both clinical trials and cadaveric studies, have been performed at the thoracic level. The mechanism of action of this block was originally thought to be due to local anesthetic spread to both dorsal and ventral rami (the latter through the costotransverse foramen) of spinal nerves resulting in a multidermatomal sensory block of the chest wall [3, 18]. However, since then other cadaver studies have been published with conflicting results (Table 1). Cadaveric studies by Adhikary et al., Vidal et al., Altinpulluk et al., Yang et al., and Elsharkawy et al. reported similar results regarding spread to both dorsal and ventral rami in either all or at least half of their injections [14–20]. However, other cadaveric studies have reported vastly different results. Ivanusic et al. reported that only 1 out of 20 thoracic ESPBs in fresh cadavers resulted in spread to ventral rami [4]. Aponte et al. reported that with six thoracic ESPBs in four fresh cadavers, no injections resulted in anterior spread to the ventral rami [26]. However, all of the aforementioned studies were performed at a thoracic vertebral level and 59% of all the blocks did not show anterior spread of injectate to the paravertebral space or ventral rami (Table 2).
Table 1.
Review of erector spinae plane block cadaver studies
| Author | Block site | No. of cadavers | No. of ESPB injections | Cadaver type | Injectate volume (ml) | Injections with anterior spread to PVS/VR |
|---|---|---|---|---|---|---|
| Elsharkawy et al. | T10–11 | 6 | 6 | Fresh | 30 | 3 of 6 |
| Altinpulluk et al. | T9 | 4 | 8 | Non-fresh | 20 | 6 of 8 |
| Vidal et al. | T4, T5, T10 | 4 | 9 | Fresh | 20 | 9 of 9 |
| Yang et al. | T5 | 10 | 10 | Fresh | 20 | 6 of 10 |
| Forero et al. | T5 | 2 | 4 | Fresh | 20 | 2 of 4 |
| Adhikary et al. | T5 | 3 | 3 | Fresh | 20 | 3 of 3 |
| Ivanusic et al. | T5 | 10 | 20 | Fresh | 20 | 1 of 20 |
| Aponte et al. | T7 | 4 | 6 | Fresh | 20 | 0 of 6 |
| De Lara González et al. | L4 | 6 | 12 | Fresh | 20 | 2 of 12 |
ESPB erector spinae plane block, PVS paravertebral space, VR ventral rami
Table 2.
Summary of ESPB cadaver studies: percentage of blocks with anterior spread
| Block location | Anterior spread | No anterior spread |
|---|---|---|
| Thoracic | ||
| Total no. of injections (n = 66) | 41% (n = 27) | 59% (n = 39) |
| Lumbar | ||
| Total no. of injections (n = 12) | 20% (n = 2) | 80% (n = 10) |
More recent literature also shows variable degree of spread pertaining to lumbar ESPB. Kokar et al. injected 10 ml of methylene blue into the fascial space between the L4 transverse process and the erector spinae muscle and found that dye spread between T12 and L5 and was associated with a wide variation of anterior and posterior spread [16].
The aim of this study was to evaluate the craniocaudal as well as the posteroanterior spread of a lumbar ESPB performed in a fresh cadaver at the transverse process of L4. Our results indicate that the injection of 20 ml of local anesthetic solution with methylene blue did not result in crossover anterior spread affecting the ventral rami of the lumbar spinal nerves. This is consistent with the results of the lumbar ESPB cadaver study by De Lara González et al. who also reported no anterior spread in 10 of 12 injections at the transverse process of L4 [17]. The study was performed in fresh cadavers and showed consistent results for the majority of injections which were evaluated using plane dissection, as well as radiographic and frozen slice models. In addition, De Lara González et al. recently published a case series of with patients who had bilateral L4 ESPBs using 20 ml of 0.2% ropivacaine prior to undergoing lumbar spine arthrodesis. The series showed that all eight patients had full 5/5 lower extremity muscle strength at multiple points from 0 through 48 h postoperatively [27]. This further supports the lack of anterior spread to ventral rami during lumbar ESPB. These results are also congruent with the study by Harbell et al. with a 20 ml volume of injectate provided in the lumbar ESPB of fresh cadavers with consistent spread to the dorsal rami and no anterior spread to ventral rami or paravertebral study.
Anatomical variance between thoracic and lumbar erector spinae planes may explain the difference in contrast spread. Unlike the thoracic erector spinae muscles, the deep musculature in the lumbar spine is contained by a thicker aponeurosis to add strength and muscular stability [28]. This dense connective tissue surrounds each muscle group individually and adheres to the transverse process, possibly mitigating penetration of injectate.
To date, most of the data surrounding lumbar ESPBs is based on case reports and observational studies on managing acute or chronic pain in the lower extremities. Most recently, Tulgar et al. reported their experience with lumbar ESPB for patients undergoing hip or proximal femur surgeries where 11 out of 12 patients achieved numerical rating score (NRS) ≤ 3 in the first 12 h postoperatively [29]. Unfortunately, the type of procedures were not controlled and other analgesic techniques routinely performed such as wound infiltration were not reported. Therefore, it is difficult to conclude efficacy. As mentioned earlier, the study by Harbell et al. injected a 20 ml volume of 0.166% methylene blue into the plane between the distal end of the L4 transverse process and erector spinae muscle which showed cephalocaudal and medial lateral spread between L3 and L5 with consistent spread to the dorsal rami, but no anterior spread to ventral rami or paravertebral space [30]. However, possible explanations include the difference in diffusion properties of local anesthetic to contrast injectate, slow absorption of local anesthetic to the ventral ramus, and the factors intrinsic to the in vivo state such as vascular distribution and local peristalsis-related medication migration. All uncertainties and inconsistencies considered, the utility of ESPB in surgeries involving the regions of lower abdomen and lower extremities is still in question. Also, the true safety of ESPB in the lumbar spine must be considered when anterior spread of local anesthesia is possible, although uncommon.
Our finding suggests that lumbar ESPB may be used in the perioperative setting where deep local anesthetic spread is discouraged such as in spine arthrodesis. In vivo studies may need to be performed to validate utilization of this technique for perioperative anesthesia for hip and groin surgeries. ESPB is a rapidly emerging procedure due to its perceived safety, technical feasibility, and possible clinical efficacy. We believe that the analgesic mechanism of lumbar ESPBs may differ from those performed in the thoracic region. Further studies are recommended to better define its mechanism of action.
Limitations
Only one cadaver assessment was performed to confirm the location of dye spread and anatomic assessment may vary by patient, volume, and speed of injectate. However, further study confirming the spread may be conducted. Areas of improvement would include standardization of controlled pressured injection to create consistency in flow and distribution of local in a standard way. Also, it would be valuable to provide a comparison of anatomic spread with standardized comparisons of volume injected and assessment of subsequent spread.
Conclusions
Our findings are consistent with current literature, and suggest that the injection spread after lumbar erector spinae nerve block performed at L4 is confined to the posterior compartment of the spine and may spare its effect on ventral nerve roots.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
All authors provided critical feedback and helped shape the research, analysis and manuscript.
Disclosures
Dr. Shaparin reports research and personal funding with Heron Therapeutics, Averitas Pharma, and AcelRX pharmaceuticals medical. Dr. Gristenko serves as a consultant for Pacira pharmaceuticals. Dr. Wahezi is a consultant for Boston Scientific and has received research funding from Vertos, Boston Scientific and Abbott. Dr. Viswanath and Dr. Kaye serve on the editorial board of Pain and Therapy. All remaining authors have no pertinent disclosures.
Compliance with Ethics and Guidelines
Ethics committee approval was not required as this was a cadaveric study with the cadaver provided by Avanos Medical Inc. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Contributor Information
Kathryn A. Breidenbach, Email: kbreidenbach@montefioreslc.org
Sayed E. Wahezi, Email: swahezi@montefiore.org
Soo Yeon Kim, Email: sykim2281@gmail.com.
Sarang S. Koushik, Email: sarang_koushik@dmgaz.org
Karina Gritsenko, Email: kgritsen@montefiore.org.
Naum Shaparin, Email: nshapari@montefiore.org.
Alan D. Kaye, Email: alan.kaye@lsuhs.edu
Omar Viswanath, Email: oviswanath@innovativepainandwellness.com.
Hall Wu, Email: Hall.Wu@med.usc.edu.
Jung H. Kim, Email: Jung.Kim@mountsinai.org
References
- 1.Dunn LK, Durieux ME, Nemergut EC. Non-opioid analgesics: novel approaches to perioperative analgesia for major spine surgery. Best Pract Res Clin Anaesthesiol. 2016;30(1):79–89. doi: 10.1016/j.bpa.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Bajwa SJ, Haldar R. Pain management following spinal surgeries: an appraisal of the available options. J Craniovertebr Junction Spine. 2015;6(3):105–110. doi: 10.4103/0974-8237.161589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41(5):621–627. doi: 10.1097/AAP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 4.Chin KJ, Dinsmore MJ, Lewis S, Chan V. Opioid-sparing multimodal analgesia with bilateral bi-level erector spinae plane blocks in scoliosis surgery: a case report of two patients. Eur Spine J. 2019 doi: 10.1007/s00586-019-06133-8. [DOI] [PubMed] [Google Scholar]
- 5.De Cassai A, Bonvicini D, Correale C, Sandei L, Tulgar S, Tonetti T. Erector spinae plane block: a systematic qualitative review. Minerva Anestesiol. 2019;85(3):308–319. doi: 10.23736/S0375-9393.18.13341-4. [DOI] [PubMed] [Google Scholar]
- 6.Gürkan Y, Aksu C, Kuş A, Yörükoğlu UH, Kılıç CT. Ultrasound guided erector spinae plane block reduces postoperative opioid consumption following breast surgery: a randomized controlled study. J Clin Anesth. 2018;50:65–68. doi: 10.1016/j.jclinane.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Ueshima H, Inagaki M, Toyone T, Otake H. Efficacy of the erector spinae plane block for lumbar spinal surgery: a retrospective study. Asian Spine J. 2019;13(2):254–257. doi: 10.31616/asj.2018.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yayik AM, Cesur S, Ozturk F, et al. Postoperative analgesic efficacy of the ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal decompression surgery: a randomized controlled study. World Neurosurg. 2019;126:e779–e785. doi: 10.1016/j.wneu.2019.02.149. [DOI] [PubMed] [Google Scholar]
- 9.Melvin JP, Schrot RJ, Chu GM, Chin KJ. Low thoracic erector spinae plane block for perioperative analgesia in lumbosacral spine surgery: a case series. Can J Anaesth. 2018;65(9):1057–1065. doi: 10.1007/s12630-018-1145-8. [DOI] [PubMed] [Google Scholar]
- 10.Almeida CR, Oliveira AR, Cunha P. Continuous bilateral erector of spine plane block at T8 for extensive lumbar spine fusion surgery: case report. Pain Pract. 2019;19(5):536–540. doi: 10.1111/papr.12774. [DOI] [PubMed] [Google Scholar]
- 11.Brandão J, Graça R, Sá M, Cardoso JM, Caramelo S, Correia C. Lumbar erector spinae plane block: successful control of acute pain after lumbar spine surgery—a clinical report. Bloqueo lumbar del plano del músculo erector de la columna: control exitoso del dolor agudo tras cirugía de la columna lumbar. Un caso clínico. Rev Esp Anestesiol Reanim. 2019;66(3):167–171. doi: 10.1016/j.redar.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Elsharkawy H, Bajracharya GR, El-Boghdadly K, Drake RL, Mariano ER. Comparing two posterior quadratus lumborum block approaches with low thoracic erector spinae plane block: an anatomic study. Reg Anesth Pain Med. 2019;rapm-2018-100147. [DOI] [PubMed]
- 13.Vidal E, Giménez H, Forero M, Fajardo M. Erector spinae plane block: a cadaver study to determine its mechanism of action. Rev Esp Anestesiol Reanim. 2018;65(9):514–519. doi: 10.1016/j.redar.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Yang HM, Choi YJ, Kwon HJ, Cho TH, Kim SH. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: a cadaveric study. Anaesthesia. 2018;73(10):1244–1250. doi: 10.1111/anae.14408. [DOI] [PubMed] [Google Scholar]
- 15.De Lara González SJ, Pomés J, Prats-Galino A, Gracia J, Martínez-Camacho A, Sala-Blanch X. Anatomical description of anaesthetic spread after deep erector spinae block at L-4. Rev Esp Anestesiol Reanim. 2019;66(8):409–416. doi: 10.1016/j.redar.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Kokar S, Ertaş A, Mercan Ö, Yildirim FG, Taştan ÖA, Akgün K. The lumbar erector spinae plane block: a cadaveric study. Turk J Med Sci. 2021 doi: 10.3906/sag-2107-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang X, Wang J, Zhang J, Kang Y, Sandeep B, Yang J. Ultrasound-guided erector spinae plane block improves analgesia after laparoscopic hepatectomy: a randomised controlled trial. Br J Anaesth. 2022;129(3):445–453. doi: 10.1016/j.bja.2022.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Forero M, Rajarathinam M, Adhikary S, Chin KJ. Continuous erector spinae plane block for rescue analgesia in thoracotomy after epidural failure: a case report. Case Rep. 2017;8(10):254–256. doi: 10.1213/XAA.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 19.Adhikary SD, Bernard S, Lopez H, Chin KJ. Erector spinae plane block versus retrolaminar block. Reg Anesth Pain Med. 2018;43(7):756–762. doi: 10.1097/AAP.0000000000000798. [DOI] [PubMed] [Google Scholar]
- 20.Altinpulluk EY, Ozdilek A, Colakoglu N, et al. Bilateral postoperative ultrasound-guided erector spinae plane block in open abdominal hysterectomy: a case series and cadaveric investigation. Rom J Anaesth Intensive Care. 2019;26(1):83–88. doi: 10.2478/rjaic-2019-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forero M, Rajarathinam M, Adhikary S, Chin KJ. Erector spinae plane (ESP) block in the management of post thoracotomy pain syndrome: a case series. J Pain. 2017;17:325–329. doi: 10.1016/j.sjpain.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Saadawi M, Layera S, Aliste J, Bravo D, Leurcharusmee P, Tran Q. Erector spinae plane block: a narrative review with systematic analysis of the evidence pertaining to clinical indications and alternative truncal blocks. J Clin Anesth. 2021;68:110063. doi: 10.1016/j.jclinane.2020.110063. [DOI] [PubMed] [Google Scholar]
- 23.Chin KJ, El-Boghdadly K, Can J. Mechanisms of action of the erector spinae plane (ESP) block: a narrative review. Anaesthesia. 2021;68(3):387–408. doi: 10.1007/s12630-020-01875-2. [DOI] [PubMed] [Google Scholar]
- 24.Ivanusic J, Konishi Y, Barrington MJ. A cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med. 2018;43(6):567–571. doi: 10.1097/AAP.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 25.Tulgar S, Kapakli MS, Senturk O, Selvi O, Serifsoy TE, Ozer Z. Evaluation of ultrasound-guided erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: a prospective, randomized, controlled clinical trial. J Clin Anesth. 2018;49:101–106. doi: 10.1016/j.jclinane.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Aponte A, Sala-Blanch X, Prats-Galino A, Masdeu J, Moreno LA, Sermeus LA. Anatomical evaluation of the extent of spread in the erector spinae plane block: a cadaveric study. Évaluation anatomique de la propagation du bloc du plan des muscles érecteurs du rachis: une étude cadavérique. Can J Anaesth. 2019;66(8):886–893. doi: 10.1007/s12630-019-01399-4. [DOI] [PubMed] [Google Scholar]
- 27.De Lara González S, BasoraMacaya M, Tió M, Martínez-Camacho A, Fuster S, Sala-Blanch X. L4 erector spinal plane block after lumbar spine arthrodesi: a case-series. Bloqueo del plano del erector espinal en L4 en cirugía de artrodesis lumbar: serie de casos. Rev Esp Anestesiol Reanim. 2019;66(10):537–542. doi: 10.1016/j.redar.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Willard FH, Vleeming A, Schuenke MD, Danneels L, Schleip R. The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat. 2012;221(6):507–553. doi: 10.1111/j.1469-7580.2012.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tulgar S, Selvi O, Senturk O, Ermis MN, Cubuk R, Ozer Z. Clinical experiences of ultrasound-guided lumbar erector spinae plane block for hip joint and proximal femur surgeries. J Clin Anesth. 2018;47:5–6. doi: 10.1016/j.jclinane.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Harbell MW, Seamans DP, Koyyalamudi V, Kraus MB, Craner RC, Langley NR. Evaluating the extent of lumbar erector spinae plane block: an anatomical study. Reg Anesth Pain Med. 2020;45(8):640–644. doi: 10.1136/rapm-2020-101523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


