Abstract
Ascochyta blight disease is a devastating disease caused by the fungal pathogen Ascochyta rabiei that threatens chickpea production around the globe. Endocytic mechanism has a significant role in fungal growth and virulence. The underlying biology of biogenesis of central component of endocytosis viz Rab5 vesicles, is not completely understood. The involvement of F-BAR domain containing protein (ArF-BAR) in various cellular processes that collectively make ArF-BAR as an important virulence determinant. Here, we report that ArF-BAR is involved in biogenesis and motility of early endosome. In the absence of ArF-BAR gene (Δarf-bar), fungal mutants exhibited reduced number of EGFP coated ArRab5 vesicles, along with the considerable reduction in their dynamics. Here, we show that ArF-BAR interacts with clathrin light chain (ArCLC), specifically with its F-BAR domain. These findings suggests the novel role of ArF-BAR in biogenesis and dynamics of early endosome. Additionally, ArF-BAR is involved in clathrin-mediated mechanism of endocytosis which is required for host infection and disease development. Identification of this pathway offers new impending targets for disease intervention in plants.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03451-5.
Keywords: Rab5, Clathrin, Fungal pathogens, Chickpea, Endocytosis
Introduction
The highly regulated and complex process of vesicular dynamics governs the interaction of the pathogen with its host environment (Rodrigues et al. 2008; Vallejo et al. 2011; Oliveira et al. 2013). Endocytosis is one of the mechanisms of vesicular trafficking meant for nutrient uptake, recycling, or degradation of cargoes, including membrane/receptor proteins, and to initiate the downstream signaling (Kalaidzidis et al. 2015; Sposini et al. 2017; Souibgui et al. 2021). All these processes synergize the polarized hyphal growth of the fungi, a prerequisite for fungal virulence and pathogenicity (Higuchi et al. 2009; Takeshita et al. 2008). The endocytic pathway progresses through different compartments, yet early endosome (EE) orchestrates the central component of the endocytic pathway (Seaman 2008). In filamentous fungi, long-range EE trafficking is required for hyphal tip growth. In addition, the motility of EE helps in protein sorting and signal transduction (Miaczynska et al. 2004). The small GTPase Rab5 plays an important regulatory role in EE biogenesis. Previous reports suggest Rab5 is indispensable for the motility of endosomes (Nielsen et al. 1999). Without Rab5, the endo-lysosomal pathway gets impaired (Zeigerer et al. 2012). We recently demonstrated that ArF-BAR localizes to EE, where it modulates the endocytosis required for fungal virulence (Sinha et al. 2021). Furthermore, ArF-BAR participates in various cellular processes viz effector secretion, actin polymerization, and hyphal penetration to mediate fungal pathogenicity. However, the rationale behind the localization of ArF-BAR to EE is unknown.
The EE comprises vesicles that get internalized in the cytosol (Grant and Donaldson 2009). Several mechanisms are involved in the internalization of endocytic cargoes (Riezman et al. 1997). However, the two most common endocytosis modes of the cells are (a) clathrin-dependent and (b) clathrin-independent endocytosis. Clathrin is a cytosolic protein composed of clathrin heavy chain (CHC) and clathrin light chain (CLC) subunits (Wu et al. 2016). The three CHC and CLC subunits assemble to form a triskelion shape structure that participates in Clathrin-mediated endocytosis from the cell surfaces (Souibgui et al. 2021). Previous studies suggest that CHC remains partially functional in the absence of CLC; however, in the absence of CLC, trimerization of CHCs is affected. Furthermore, erstwhile reported that CLC associates with membranes to facilitate vesicle budding (Huang et al. 1997). Thus far, most F-BAR protein characterized from animal systems acts as nucleators of clathrin-mediated endocytosis (Henne et al. 2010). In mammalian cells, clathrin-mediated endocytosis is well characterized (Barrias et al. 2019); however, in filamentous fungi, the function of clathrin remains elusive (Souibgui et al. 2021).
Chickpea (Cicer arietinum L.) is the second most widely grown food legume after soybean (Varshney et al. 2013). Ascochyta rabiei (Pass.) Labr. (telomorph Didymella rabiei) is the most devastating necrotrophic phytopathogen for chickpea. A. rabiei is a filamentous necrotroph that infects the foliar parts of the plant, resulting in Ascochyta Blight (AB) disease in chickpea (Pandey et al. 1987). Eventually, the availability of genome sequence of A. rabiei (Verma et al. 2016) has made A. rabiei an efficient model system for understanding the pathogenicity and virulence of the fungus.
In the present study, we aimed to identify the relevance of ArF-BAR localization with EE. In advancement to our recent findings from Sinha et al. (2021), where ArF-BAR has an imperative role in fungal pathogenicity, here we identify that ArF-BAR is necessary for biogenesis and motility of EE. We provide evidence for the significant difference in the number and dynamics of GFP decorated Rab5 coated EE in the absence of ArF-BAR (Δarf-bar). In addition, we also identify the clathrin gene from A. rabiei, where ArF-BAR interacts with ArCLC. Overall, findings from this study broaden the understanding of the endocytic mechanism in filamentous phytopathogen A. rabiei, where ArF-BAR is involved in clathrin-mediated endocytosis, and further, it regulates the biogenesis and motility of EE to promote fungal infection.
Materials and methods
Fungal strains and growth conditions.
Ascochyta rabiei (ArD2; ITCC No. 4638) virulent strain was obtained from IARI, New Delhi. This mating type 2 isolate used in this study was maintained as wild-type fungi and used for genetic modifications for research work. In addition, the fungus was routinely sub-cultured on potato dextrose agar (PDA; Difco Laboratories, pH 5.2–5.5) at 22 °C, as described in Sinha et al. 2021. A list of fungal strains used in this study is listed in Table S1.
Yeast two-hybrid assays
The interactions between ArF-BAR, F-BAR domain, SH3, and ArCLC, in yeast cytoplasm were performed using the split-ubiquitin-based DUALhunter system (Dualsystems Biotech). First, the ORFs were cloned into pGDHB1 and pGPR3-N vectors at NotI and AscI restriction sites. A list of Oligonucleotides used for cloning are listed in Table S2. Then, using the EZ‐Yeast transformation kit (MP Biomedicals, USA), the cloned plasmids were co-transformed into NMY51 strain along with the necessary controls. Further, yeast strains were plated on SD‐L/‐W plates. The plating of yeast clones on required synthetic media to check protein–protein interactions in yeast was done as described previously (Kumar et al. 2018). All the interactions were verified by three independent experiments.
Confocal laser scanning microscopy
TCS SP8 confocal laser scanning microscopes (Leica Microsystems, Germany) were used for confocal microscopic studies. The conidia from the respective fungal strains were harvested in 1 mL sterile distilled water from 15-day-old fungal mycelia grown on PDA plates. The conidial suspension was filtered through Mira cloth. Ten microliters (1 × 106 conidia/mL) of suspension was kept on a sterile glass coverslip and allowed to grow under optimal condition for 24 h in the dark, as described in Sinha et al. (2021). Live-cell imaging was performed in TCS SP8, and the mobility of chimeric fluorescent proteins was determined. Image processing was accomplished by Leica Application Suite software and ImageJ. Kymograph and average speed of the moving particles were determined by KymoButler (Jakobs et al. 2019).
Bioinformatics analysis
The corresponding gene and protein sequences were retrieved from the NCBI server (http://www.ncbi.nlm.nih.gov). First, multiple sequence alignment was performed by PROMALS3D using default parameters, and then alignment was visualized with visualization software Jalview. Next, phylogenetic analysis was performed with MEGAX software using the neighborhood-joining method, having a bootstrap value of 1000. Finally, conservation in the CLC domain was predicted using Batch CD-search from NCBI, and the representative image was made using TBtools software. Domain architecture of the protein was determined by InterPro Scan and Motif Finder online search tool.
Results
ArF-BAR promotes biogenesis and dynamics of early endosomes.
ArF-BAR knockout mutant (Δarf-bar) greatly exhibits impaired endocytosis compared to endocytosis in A. rabiei, wild type (WT) (Sinha et al. 2021). Therefore, to investigate the role of ArF-BAR in endosomal biogenesis, ArRab5 was translationally fused to EGFP. The ArRab5::EGFP fusion construct was transformed simultaneously in WT and Δarf-bar, using Agrobacterium-mediated transformation (ATMT). Interestingly, the Δarf-bar transformants expressing ArRab5::EGFP showed a significantly smaller number of ArRab5 subpopulations than WT (Fig. 1a).
Fig. 1.
ArF-BAR regulates the biogenesis and motility of early endosome. a Confocal image showing EGFP-ArRab5 coated early endosomes in WT and Δarf-bar. Difference in the number of GFP coated Rab5 vesicles were observed in hyphae of two different fungal transformants. More number of ArRab5 punctate were observed in WT as compared to Δarf-bar. Images were optically sectioned, Z-stack images having 1 µm step size were captured. b Confocal images of migrating ArRab5 decorated with EGFP, in WT and Δarf-bar. Arrow head indicates the temporal localization of early endosomes. c Kymographs of moving Rab5 endosomes in WT and Δarf-bar. Sale Bar represents 10 µm in length
The EGFP-tagged ArRab5 punctate structures were visualized using time-lapse microscopy to explore the possible role of ArF-BAR in endosomal dynamics. Here, we observed that ArRab5::EGFP punctate structures exhibited characteristic bidirectional motility along the WT hypha (Fig. 1b, Video. S1), and this motility was significantly reduced in Δarf-bar (Fig. 1b, Video. S2). The average dynamic rates of ArRab5 motility in WT and Δarf-bar were 1.2 and 0.19 µm/s, respectively, as derived from a kymograph along the growth axis (Fig. 1c). Overall, these experiments confirmed the role of ArF-BAR in endocytosis, mediated through biogenesis and the dynamics of EE.
Clathrin light chain domain and phylogenetic analysis
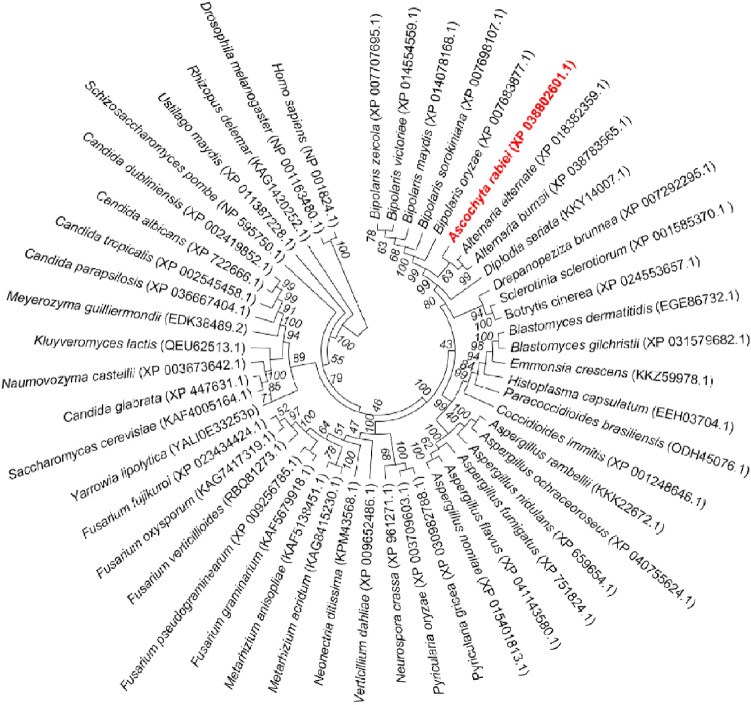
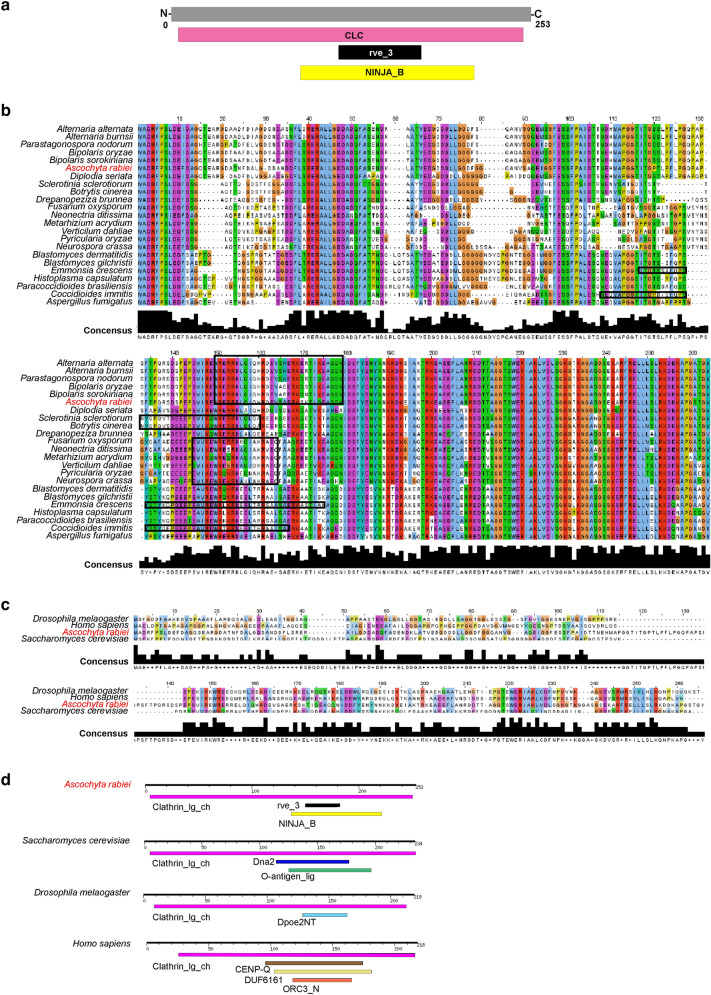
To gain insights into the CLC domain of A. rabiei, initially, we identified gene encoding clathrin light chain from WT (ArCLC) through the BLAST search tool, using previously characterized Clc from Saccharomyces cerevisiae. Phylogenetic relationships among selected fungal pathogens and other eukaryotes suggest that ArCLC shares closer homology with protein sequences of closely associated pathogenic fungi (Fig. 2). Domain architecture prediction suggests the presence of single Clathrin-light chain domain (5–250 amino acid) in CLC protein from A. rabiei. However, integrase core domain (rve_3; 150–179 amino acids) and putative nuclear localization signal (NINJA_B; 137–221 amino acids) are present within the Clathrin-light chain domain (Fig. 3a). Homology-based sequence alignment suggests that CLC are highly conserved among fungi of various species (Fig. 3b). The Integrase core domain (rve_3) is exclusively conserved in majority of pathogenic fungi (Fig. 3b). ArCLC shares approximately 32.13%, 24.50%, and 18.80% sequence identity with widely characterized CLCs from distantly related species S. cerevisiae, D. melanogaster, and H. sapiens, respectively (Fig. 3c). The domain organization of CLC protein from different organisms including S. cerevisiae, D. melanogaster, and H. sapiens unravels the presence of single CLC domain similar to A. rabiei, however none of the CLC protein contain the sequence of rve_3 (Fig. 3d).
Fig. 2.
Evolutionary relationships of ArCLC with other homologous proteins among different organisms. The phylogenetic tree obtained through the neighborhood-joining method, based on the analysis of protein sequences isolated from various pathogenic fungi, yeast, D. melanogaster, and H. sapiens, constructed using MEGAX. Protein IDs are included in parentheses, whose sequence were used to construct phylogeny
Fig. 3.
Multiple sequence alignment of ArCLC protein with its homologs from various organisms. a Schematic representation of ArCLC protein. The 5–250 amino-acid protein has Clathrin-light chain (CLC) domain (pink), 150–179 amino-acid has Integrase core domain (rve_3) (black), and 137–221 amino acid has putative nuclear localization signal (yellow). b Multiple sequence alignment shows that CLC protein is highly conserved in different fungal species. The integrase core domain is marked in the box (black). c Multiple sequence alignment showing the conservation of ArCLC protein with well characterized CLC protein from S. cerevisiae, D. melanogaster, and H. sapiens. The alignment of the protein was determined by multiple sequence and structure alignment program PROMALS3D using default parameters. The alignment was visualized with visualization software Jalview. Color code for sequence conservation varies from blue (least conserved) to red (highly conserved). Shaded box below the alignment indicates the consensus sequence or the degree of conservation among the proteins. d Domain organization of CLC protein from S. cerevisiae, D. melanogaster, and H. sapiens
ArF-BAR interacts with the clathrin light chain
The involvement of ArF-BAR protein in fungal virulence via endocytosis encouraged us to investigate for possible correlation between ArF-BAR and clathrin-mediated endocytosis. To determine the role of ArF-BAR in clathrin-mediated endocytosis, we checked for the probable protein–protein interactions using the Yeast-2-hybrid (Y2H) system. Yeast cells transformed with ArF-BAR and ArCLC constructs were similar to positive control when grown on auxotrophic selection media lacking amino acids like leucine, tryptophan, histidine, and adenine (SD/-L-T-H-A). Thus Y2H result established the positive interaction of ArF-BAR protein with ArCLC (Fig. 4a). As mentioned previously, ArCLC contains single CLC domain. So far, this domain is the main functional domain known in endocytosis. CLC domain majorly covers the entire length of ArCLC protein. Therefore, full-length ArCLC protein was used for interaction. ArF-BAR protein contains the F-BAR domain, C1 domain, and two consecutive SH3 domains (Sinha et al. 2021). Thus, to ascertain the involvement of specific domains of ArF-BAR in protein–protein interaction, truncated expressions constructs of ArF-BAR were generated. Initially, the interaction between the SH3 domain and ArCLC was analyzed. Here, yeast cells failed to grow on SD/-L-T-H-A (Fig. 4b). Furthermore, when protein–protein interaction between the F-BAR domain and ArCLC was assayed, growth of yeast cells was found to be similar to that of ArF-BAR (Fig. 4c). Thus, the Y2H interaction study reveals that ArF-BAR interacts with ArCLC through its F-BAR domain. Overall, these results suggest the role of ArF-BAR in clathrin-mediated endocytosis.
Fig. 4.
ArF-BAR interacts ArCLC via F-BAR domain. a Split-ubiquitin based yeast two-hybrid assay to check protein–protein interaction between ArF-BAR and ArCLC in yeast cytoplasm. The yeast strain NMY-51 was co-transformed with bait and prey. Growth of yeast cells on interaction selective media QDO (SD/-L-W-A-H), suggests positive interaction between ArF-BAR and ArCLC. LargeT and Δp53 are used as positive control, while the empty bait and prey vector was used as negative control. pAI-Alg5 and pDL2-Alg5 are used to check the transactivation property. b Yeast cells failed to grow on QDO, as compared to DDO (SD/-L-W), when they were co-transformed with ArCLC and SH3 domains [SH3(ArF-BAR); 508–760 amino acids] of ArF-BAR, suggesting SH3 of ArF-BAR has no role in interaction with ArCLC. c Yeast cells was observed on QDO when they were co-transformed with F-BAR domain of ArF-BAR and ArCLC, suggesting ArF-BAR interacts ArCLC via F-BAR domain. The representative images were captured after 48 h of spotting of yeast cells
Discussion
The membrane curvature generation is regulated by BAR superfamily proteins (Almeida-Souza et al. 2018). ArF-BAR was previously reported as a potential candidate for fungal pathogenesis, where it is involved in hyphal architecture maintenance, hyphal penetration, effector secretion, actin polymerization, and endocytosis (Sinha et al. 2021). In the absence of ArF-BAR (Δarf-bar), we showed a decrease in GFP-tagged ArRab5 coated EE vesicles. The current study strengthens the mechanism underlying endosome formation, their dynamics through membrane curvature generation, and their role in the pathogenesis of necrotrophic fungi, A. rabiei. ArF-BAR emerges from this study as an important regulator of EE biogenesis and motility. Motility of EE is the common phenomenon of eukaryotes that helps in sorting endocytic cargoes (Lemmon and Traub 2000). Additionally, it participates in various cellular mechanisms like cell signaling (Sorkin and von Zastrow 2002), effector secretion (Bielska et al. 2014), and cytokinesis (Carlton and Martin-serrano 2007), cell polarity, and migration (Emery and Knoblich 2006). The nucleus is distantly located in filamentous fungi from the growing hyphal tip (Etxebeste and Espeso 2016). Therefore, in fungi, passive diffusion is insufficient for long-range signaling; here, constantly moving EE helps overcome the challenge of long-range signal transduction (Miaczynska et al. 2004; Steinberg 2007). Furthermore, motile EE is required for hyphal growth and membrane recycling (Wedlich-Söldner et al. 2000; Fuchs et al. 2006). Furthermore, the bidirectional movement of EE participates in the distribution of cellular organelles and entire polysomes throughout the fungal hyphae (Schuster et al. 2011; Higuchi et al. 2014). Thus far, this evidence suggests that the dynamicity of EE is required for fungal virulence, and ArF-BAR servers as an important candidate for regulating the biogenesis and function of EE.
The mechanism of endocytosis has a significant role in the physiology and pathogenicity of fungus (Sinha et al. 2021). In eukaryotes, clathrin-mediated endocytosis is well characterized for internalization pathways (Schultzhaus and Shaw 2015). However, despite the significance of endocytosis in the growth of filamentous fungi during plant-pathogen interaction, the role of clathrin in fungal pathogenesis is poorly understood (Souibgui et al. 2021). Domain architecture of CLC protein signifies that the Integrase core domain is conserved in majority of the CLC protein of pathogenic fungi. Interestingly, this integrase domain is absent in the CLC protein of different organisms. Integrase enzyme is well for integrating a copy viral DNA into its target host chromosome (Dyda et al. 1994). The presence of integrase core domain along with the signal sequence required for nuclear localization in CLC protein in majority of the pathogenic fungi, indicates the probable existence of fungal pathogenicity via Clathrin-mediated endocytic mechanism. The presence CLC domain in S. cerevisiae, D. melanogaster, and H. sapiens suggests the conserved function of CLC protein from yeast to mammals. However, during the course of evolution different regions have been acquired in CLC domain for different functions. In this study, the interaction of CLC with ArF-BAR shed light on the existence of clathrin-mediated endocytosis in A. rabiei via previously well-characterized ArF-BAR. Thus far, this work has paved the way for understanding the detailed mechanism and significance of this interaction.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
All the authors gratefully acknowledge a research grant from Department of Biotechnology, Government of India (File No: BT/PR10605/PBD/16/791/2008) and a core grant from National Institute of Plant Genome Research (NIPGR), New Delhi, India for funding this work. Authors thank Central Instrumental Facility of NIPGR for utilizing various instruments. AS acknowledge Council of Scientific & Industrial Research, Government of India for fellowship to carry out the research work. MS acknowledge University Grant Commission, Government of India for fellowship to carry out the research work.
Data availability
The datasets generated during the current study is included in this article.
Declarations
Conflict of interest
Authors have no competing interest to declare that are relevant to the content of this article.
References
- Almeida-Souza L, Frank RAW, García-Nafría J, Colussi A, Gunawardana N, Johnson CM, Yu M, Howard G, Andrews B, Vallis Y, et al. A Flat BAR protein promotes actin polymerization at the base of clathrin-coated pits. Cell. 2018 doi: 10.1016/j.cell.2018.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrias E, Reignault L, de Carvalho TMU, de Souza W. Clathrin coated pit dependent pathway for Trypanosoma cruzi internalization into host cells. Acta Trop. 2019;199:105057. doi: 10.1016/j.actatropica.2019.105057. [DOI] [PubMed] [Google Scholar]
- Bielska E, Higuchi Y, Schuster M, Steinberg N, Kilaru S, Talbot NJ, Steinberg G. Long-distance endosome trafficking drives fungal effector production during plant infection. Nat Commun. 2014;5:6–10. doi: 10.1038/ncomms6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton JG, Martin-serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT Machinery. Science. 2007;316:1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- Dyda F, Hickman AB, Jenkins TM, Engelman A, Craigie R, Davies DR. Crystal structure of the catalytic domain of HIV-1 integrase: similarity to the polynucleotidyl transferases. Science. 1994;266:1981–1986. doi: 10.1126/science.7801124. [DOI] [PubMed] [Google Scholar]
- Emery G, Knoblich JA. Endosome dynamics during development. Curr Opin Cell Biol. 2006;18:407–415. doi: 10.1016/j.ceb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Etxebeste O, Espeso EA. Neurons show the path: tip-to-nucleus communication in filamentous fungal development and pathogenesisa. FEMS Microbiol Rev. 2016;40:610–624. doi: 10.1093/femsre/fuw021. [DOI] [PubMed] [Google Scholar]
- Fuchs U, Hause G, Schuchardt I, Steinberg G. Endocytosis is essential for pathogenic development in the corn smut fungus Ustilago maydis. Plant Cell. 2006;18:2066–2081. doi: 10.1105/tpc.105.039388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, Mcmahon HT. Europe PMC Funders Group FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328:1281–1284. doi: 10.1126/science.1188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi Y, Shoji JY, Arioka M, Kitamoto K. Endocytosis is crucial for cell polarity and apical membrane recycling in the filamentous fungus Aspergillus oryzae. Eukaryot Cell. 2009;8:37–46. doi: 10.1128/EC.00207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi Y, Ashwin P, Roger Y, Steinberg G. Early endosome motility spatially organizes polysome distribution. J Cell Biol. 2014;204:343–357. doi: 10.1083/jcb.201307164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KM, Gullberg L, Nelson KK, Stefan CJ, Blumer K, Lemmon SK. Novel functions of clathrin light chains: clathrin heavy chain trimerization is defective in light chain-deficient yeast. J Cell Sci. 1997;110:899–910. doi: 10.1242/jcs.110.7.899. [DOI] [PubMed] [Google Scholar]
- Jakobs MA, Dimitracopoulos A, Franze K. Kymobutler, a deep learning software for automated kymograph analysis. Elife. 2019;8:e42288. doi: 10.7554/eLife.42288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaidzidis I, Miaczynska M, Brewinska-Olchowik M, Hupalowska A, Ferguson C, Parton RG, Kalaidzidis Y, Zerial M. APPL endosomes are not obligatory endocytic intermediates but act as stable cargo-sorting compartments. J Cell Biol. 2015;211:123–144. doi: 10.1111/nan.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Purayannur S, Kaladhar VC, Parida SK, Verma PK. mQTL-seq and classical mapping implicates the role of an AT-HOOK MOTIF CONTAINING NUCLEAR LOCALIZED (AHL) family gene in Ascochyta blight resistance of chickpea. Plant Cell Environ. 2018;41:2128–2140. doi: 10.1111/pce.13177. [DOI] [PubMed] [Google Scholar]
- Lemmon SK, Traub LM. Sorting in the endosomal system in yeast and animal cells. Curr Opin Cell Biol. 2000;12:457–466. doi: 10.1016/s0955-0674(00)00117-4. [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Pelkmans L, Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol. 2004;16:400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M. Rab5 regulates motility of early endosomes on microtubules. Nat Cell Biol. 1999;1:376–382. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- Oliveira DL, Rizzo J, Joffe LS, Godinho RMC, Rodrigues ML. Where do they come from and where do they go: candidates for regulating extracellular vesicle formation in fungi. Int J Mol Sci. 2013;14:9581–9603. doi: 10.3390/ijms14059581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey BK, Singh US, Chaube HS. Mode of infection of ascochyta blight of chickpea caused by Ascochyta rabiei. J Phytopathol. 1987;119:88–93. doi: 10.1111/j.1439-0434.1987.tb04387.x. [DOI] [Google Scholar]
- Riezman H, Woodman PG, Van Meer G, Marsh M. Molecular mechanisms of endocytosis. Cell. 1997;91:731–738. doi: 10.1016/S0092-8674(00)80461-4. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, Casadevall A. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultzhaus ZS, Shaw BD. Endocytosis and exocytosis in hyphal growth. Fungal Biol Rev. 2015;29:43–53. doi: 10.1016/j.fbr.2015.04.002. [DOI] [Google Scholar]
- Schuster M, Kilaru S, Fink G, Collemare J, Roger Y, Steinberg G. Kinesin-3 and dynein cooperate in long-range retrograde endosome motility along a non-uniform microtubule array. Mol Biol Cell. 2011;22:3645–3657. doi: 10.1091/mbc.e11-03-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MNJ. Membrane traffic in the secretory pathway: endosome protein sorting: motifs and machinery. Cell Mol Life Sci. 2008;65:2842–2858. doi: 10.1007/s00018-008-8354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M, Shree A, Singh K, Kumar K, Singh SK, Kumar V, Verma PK. Modulation of fungal virulence through CRZ1 regulated F-BAR-dependent actin remodeling and endocytosis in chickpea infecting phytopathogen Ascochyta rabiei. PLoS Genet. 2021;17:e1009137. doi: 10.1371/journal.pgen.1009137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- Souibgui E, Bruel C, Choquer M, de Vallée A, Dieryckx C, Dupuy JW, Latorse MP, Rascle C, Poussereau N. Clathrin is important for virulence factors delivery in the necrotrophic fungus Botrytis cinerea. Front Plant Sci. 2021;12:1–15. doi: 10.3389/fpls.2021.668937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sposini S, Jean-Alphonse FG, Ayoub MA, Oqua A, West C, Lavery S, Brosens JJ, Reiter E, Hanyaloglu AC. Integration of GPCR signaling and sorting from very early endosomes via opposing APPL1 mechanisms. Cell Rep. 2017;21:2855–2867. doi: 10.1016/j.celrep.2017.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G. On the move: endosomes in fungal growth and pathogenicity. Nat Rev Microbiol. 2007;5:309–316. doi: 10.1038/nrmicro1618. [DOI] [PubMed] [Google Scholar]
- Takeshita N, Higashitsuji Y, Konzack S, Fischer R. Apical sterol-rich membranes are essential for localizing cell end markers that determine growth directionality in the filamentous fungus Aspergillus nidulans. Mol Biol Cell. 2008;19:339–351. doi: 10.1091/mbc.E07-06-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo MC, Matsuo AL, Ganiko L, Medeiros LCS, Miranda K, Silva LS, Freymüller-Haapalainen E, Sinigaglia-Coimbra R, Almeida IC, Puccia R. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly Immunogenic α-galactosyl epitopes. Eukaryot Cell. 2011;10:343–351. doi: 10.1128/EC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney RK, Song C, Saxena RK, Azam S, Yu S, Sharpe AG, Cannon S, Baek J, Rosen BD, Tar’an B, et al. Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat Biotechnol. 2013;31:240–246. doi: 10.1038/nbt.2491. [DOI] [PubMed] [Google Scholar]
- Verma S, Gazara RK, Nizam S, Parween S, Chattopadhyay D, Verma PK. Draft genome sequencing and secretome analysis of fungal phytopathogen Ascochyta rabiei provides insight into the necrotrophic effector repertoire. Sci Rep. 2016;6:1–14. doi: 10.1038/srep24638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Söldner R, Bölker M, Kahmann R, Steinberg G. A putative endosomal t-SNARE links exo- and endocytosis in the phytopathogenic fungus Ustilago maydis. EMBO J. 2000;19:1974–1986. doi: 10.1093/emboj/19.9.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Majeed SR, Evans TM, Camus MD, Wong NML, Schollmeier Y, Park M, Muppidi JR, Reboldi A, Parham P, et al. Clathrin light chains’ role in selective endocytosis influences antibody isotype switching. Proc Natl Acad Sci USA. 2016;113:9816–9821. doi: 10.1073/pnas.1611189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigerer A, Gilleron J, Bogorad RL, Marsico G, Nonaka H, Seifert S, Epstein-Barash H, Kuchimanchi S, Peng CG, Ruda VM, et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature. 2012;485:465–470. doi: 10.1038/nature11133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study is included in this article.