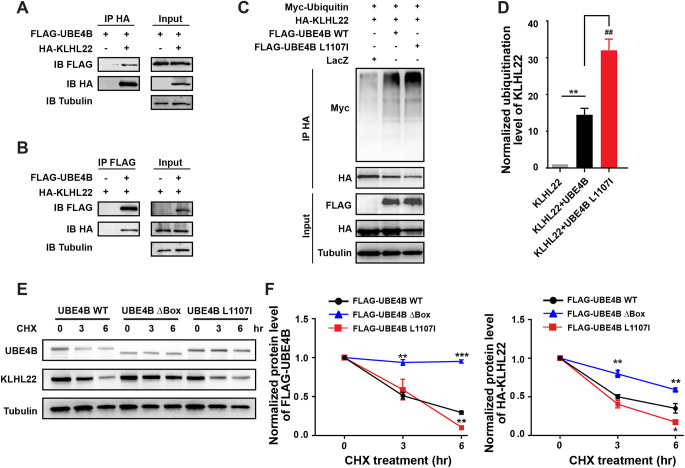
Fig. 3.
UBE4B promotes ubiquitylation and degradation of KLHL22. (A,B) Reciprocal co-IP of FLAG-UBE4B and HA-KLHL22 in HEK293T cells. Cells were treated with the proteasome inhibitor bortezomib (5 µM) for 4 h and lysed for immunoprecipitation with either anti-HA antibody (A) or anti-FLAG antibody (B). γ-Tubulin was used as the loading control of inputs. Results are representative of three independent experiments. (C) UBE4B WT or L1107I (hyperactive mutant) were co-transfected with KLHL22 and Myc-ubiquitin into HEK293T cells as indicated. pcDNA6b-lacZ was used as control. Cells were pre-treated with bortezomib for 4 h before collection for immunoprecipitation with anti-HA antibody. Polyubiquitylation signals of KLHL22 were detected by anti-Myc immunoblotting. (D) Quantification of normalized polyubiquitylation levels of HA-KLHL22 in HEK293T cells overexpressing UBE4B WT or L1107I. KLHL22 polyubiquitylation signals detected by anti-Myc antibody were normalized to the total KLHL22 proteins immunoprecipitated by anti-HA antibody. Then this ratio in UBE4B WT or L1107I-expressing cells was normalized to that in cells transfected with the pcDNA6b-lacZ. Three independent immunoblots were analyzed. Data represent mean±s.e.m. **P<0.01, ##P<0.01 (relative to the indicated controls; unpaired, two-tailed Student's t-test). (E) UBE4B-knockout Neuro2A cells were transfected with HA-KLHL22 and FLAG-UBE4B WT, ΔBox or L1107I. Cells were treated with 1 μM cycloheximide (CHX) for 0, 3 or 6 h before collection. Equal amounts of cell lysates were immunoblotted with the indicated antibodies. (F) Normalized protein levels of FLAG-UBE4B and HA-KLHL22 at different time points as shown in E. Three independent experiments were quantified. Data represent mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001 (relative to FLAG-UBE4B WT-expressing cells at each corresponding time point; one-way ANOVA).