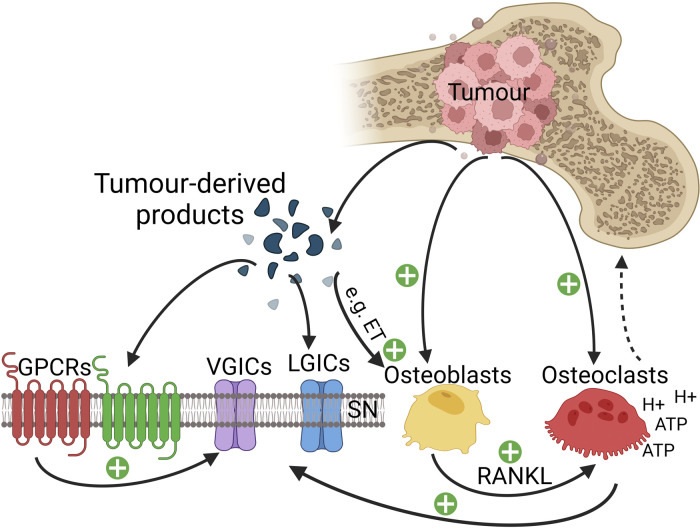
Figure 1.
Cellular interactions in the bone microenvironment in CIBP. Tumour cells release endothelin (ET), which interacts with osteoblasts via their appropriate receptors to stimulate the proliferation of osteoblasts. Activated osteoblasts release receptor activator of nuclear factor-kappa-Β ligand (RANKL), which serves as a signal for osteoclast proliferation and maturation to enhance osteoclast-mediated bone matrix destruction. Osteoclasts generate adenosine triphosphate (ATP) and acidosis by releasing protons, resulting in the activation of various receptors and ligand-gated ion channels (LGICs) like P2X receptors, transient receptor potential V1 receptors and acid-sensing ion channels type 3 expressed on bone innervating sensory neurons. Tumour cells, stromal cells and activated immune cells release a variety of mediators (such as endothelin, the nerve growth factor, protons, and pro-inflammatory cytokines) that activate their respective receptors expressed on sensory neurons and thereby initiate the detection of noxious stimuli. GPCRs can sometimes indirectly sensitise various voltage-gated ion channels (VGICs) expressed on sensory neurons leading to a further potentiation of nociceptive signalling to the spinal cord. Osteolytic cancers (like breast cancer) activate osteoclasts, while osteosclerotic cancers (like prostate cancer) activate osteoblasts leading to a further potentiation of pain signal transmission.