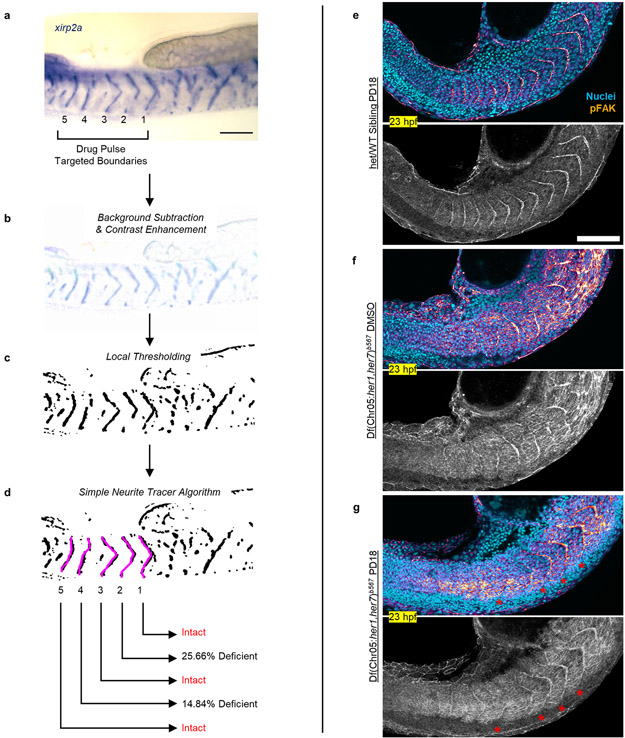
Extended Data Figure 7: Characterization of somite boundaries following pulsatile drug treatments.
a, xirp2a ISH staining marks somite boundaries of a clock-deficient mutant treated 5× pulses of 30 μM SU5402. A short working distance objective is used to minimize out of plane staining from the other side of embryos. Drug targeted somites are numbered 1 – 5 from anterior to posterior. b-d, Boundary intactness and deficiency are calculated after light background subtraction and contrast enhancement (b) and local thresholding of the image for binarization (c). Trace LOIs (magenta) running in between the ventral and dorsal ends of somitic tissue are drawn by automated simple neurite tracer algorithm following xirp2a boundary staining (d). Percentage occupancy along the trace LOIs (average signal) identifies intact boundaries and measures deficiency level of non-intact boundaries. e-g, Phosphorylated focal adhesion kinase (pFAK, gem LUT from dark purple to burnt orange) and cell nuclei (cyan) staining of embryos fixed at 23 hpf (4 h after the 5th pulse). Clock-intact siblings treated with 600 nM PD184352 (n=37 embryos over 2 independent experiments, e), and clock-deficient mutants treated with DMSO (N=2, n=13, f) or 600 nM PD184352 (n=33 embryos over 2 independent experiments, g). Red stars show induced somite boundary epithelization driven by pulsatile treatments. Bottom images are pFAK staining alone shown in grayscale. Scale bars are 100 μm.