| 1 |
Boltorn® H40 and poly(vinylpyridine) nanogel |
|
CuAAC assisted in covalent bond formation resulting in the chemically cross-linked nanogels comprising hyperbranched dendrimer (H40-PCL) with PVP as a crosslinker through mini emulsion polymerization |
pH-sensitive nanogel utilized in the release of a hydrophobic anti-cancer medication |
128
|
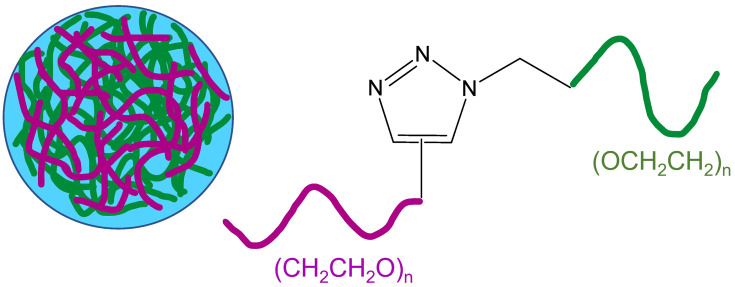
| 2 |
Nanogel formed through diacetylenic-based glycolipids |
|
The presence of a 1,2,3-triazole ring between the hydrophobic and hydrophilic part of the diacetylene-based glycolipids resulted in their self-organization into 1D-nanotubes, and at higher concentrations, these bundled nanotubes crosslinked to form the nanogels |
Nanocontainer for cytotoxic topotecan drug incorporation and its site-specific release |
129
|
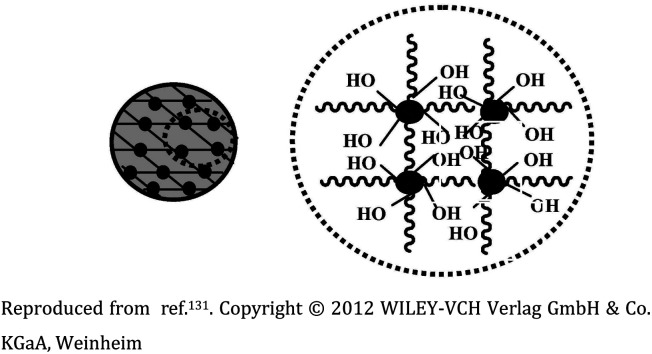
| 3 |
Poly(ethylene glycol) (PEG) monomers-based nanogel |
|
Alkyne and azide functionalized PEG monomers were crosslinked via CuAAC to synthesize the nanogel and the polymerization was subsequently ceased by the addition of a Cu-chelating agent |
The nanogel coatings efficiently exhibit short-term protein resistance, encompassing superior surfaces which could be well utilized for in vitro diagnostics |
130
|
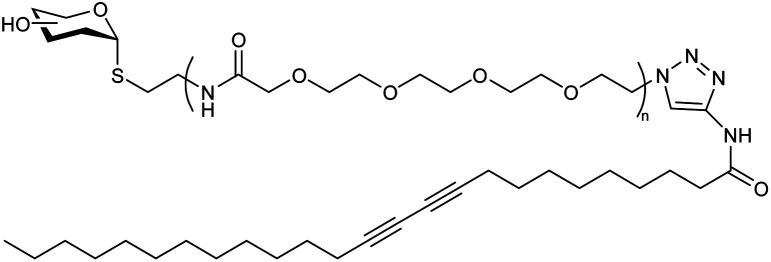
| 4 |
Ga-porphyrin-OH nanogel based on PEG |
|
Fluorescent nanogel synthesized via reverse (mini)emulsion-CuAAC having uniform size |
Applicability in near-infrared (NIR) imaging due to their fluorescence and cell affinity |
131
|
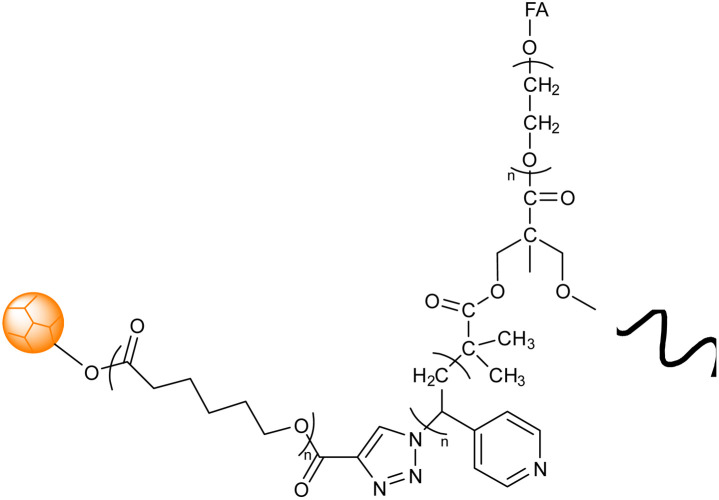
| 5 |
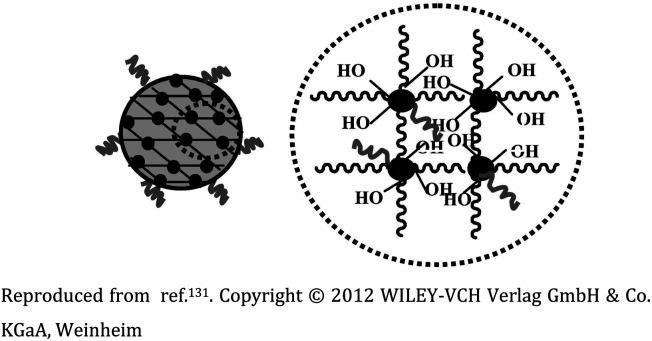
Ga-porphyrin-FA nanogel based on PEG |
|
The diameter of nanogels ranged between 30–120 nm, with an emission maximum between 700 and 800 nm. Applicability in near-infrared (NIR) imaging due to their fluorescence and cell affinity |
Folate immobilization on the surface to target tumor cells for cell imaging and drug release |
131
|
| 6 |
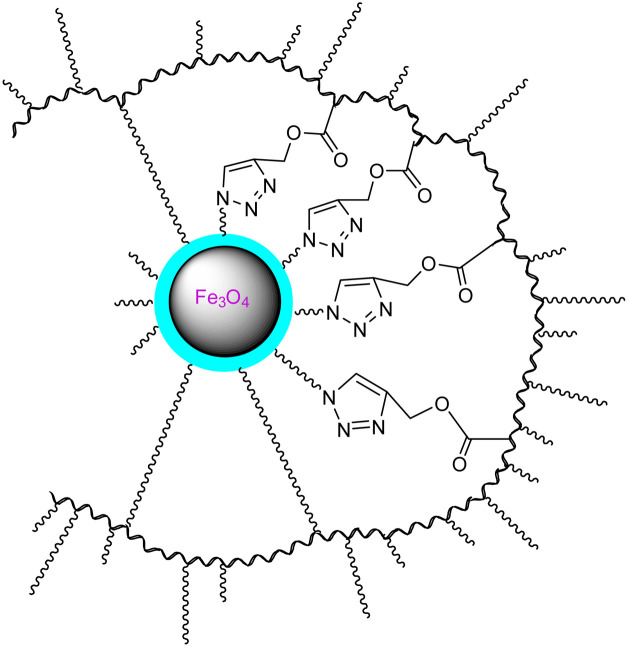
Hydroxyethyl methacrylate (HEMA), acrylated methyl ether poly (ethylene glycol) (ACMPEG), and Fe3O4 based nanogel |
|
Click chemistry and surfactant-free emulsion photopolymerization in combination to produce amphiphilic nanogels from vinylated SPIONs/HEMA/PEG |
Carrier for the anti-cancer drug quercetin |
132
|
| 7 |
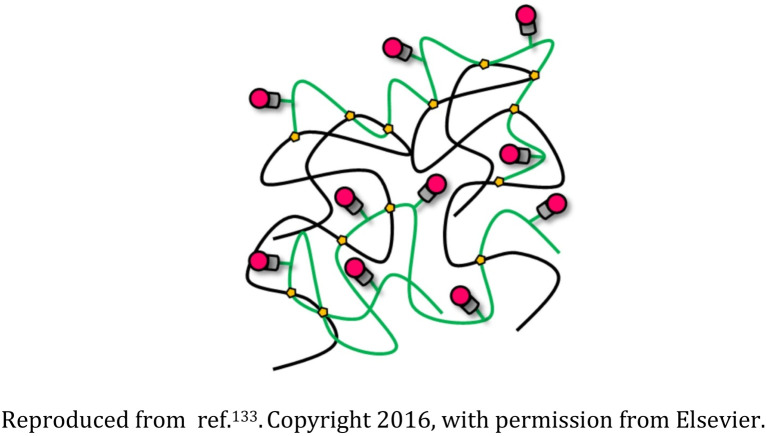
Polyethylene glycol (PEG), polyethyleneimine (PEI), and rhodamine-based nanogel |
|
The nanostructures were biodegradable and appropriate for transporting drugs or genetic material and also could be tracked as they interacted with the cells |
Drug delivery devices for sustained release of the succinimide anticonvulsant drug ethosuximide |
133
|
| 8 |
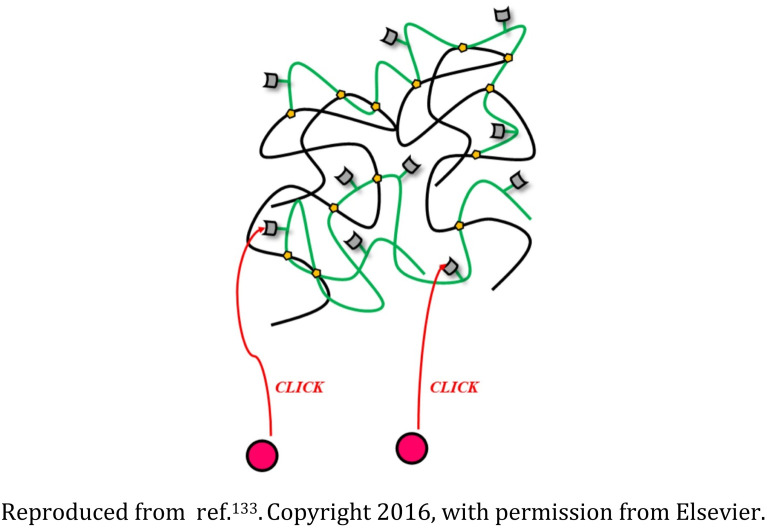
Polyethylene glycol (PEG), polyethyleneimine (PEI), and rhodamine-based nanogel |
|
The nanostructures were biodegradable and appropriate for transporting drugs or genetic material and also could be tracked as they interacted with the cells |
Drug delivery devices for sustained release of the succinimide anticonvulsant drug ethosuximide |
133
|
| 9 |
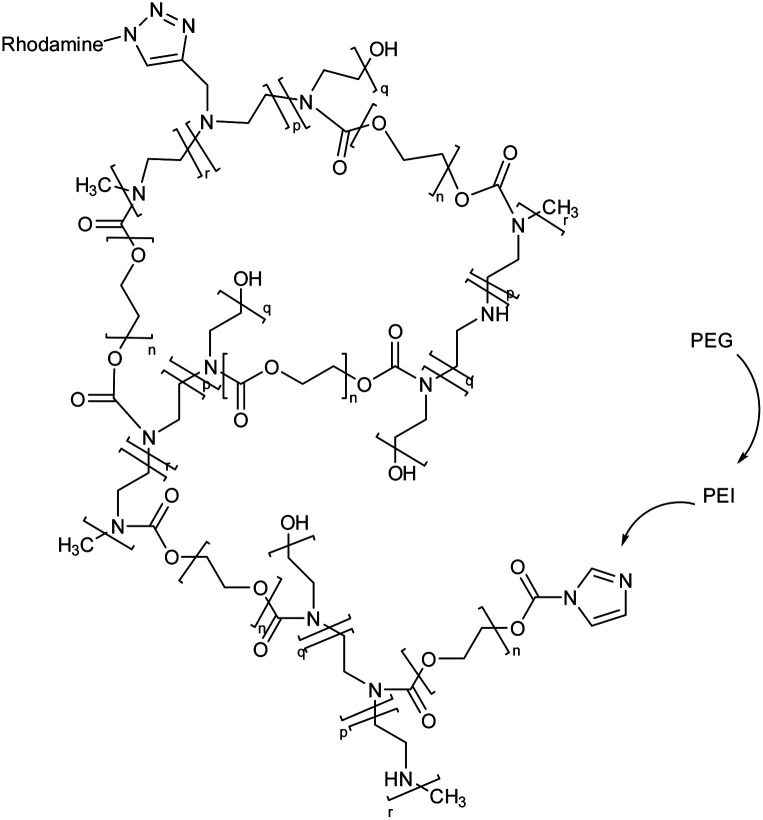
Polyethylene glycol (PEG), and polyethyleneimine (PEI) based nanogel framed via rhodamine |
|
The potential of the nanogels to enhance or decrease microglia internalization was evaluated after they were coated with various concentrations of PEG monomethyl ether |
Transportation of drugs or genes into the microglia environment, enhancing their therapeutic efficacy for reduction of microglia internalization |
134
|
| 10 |
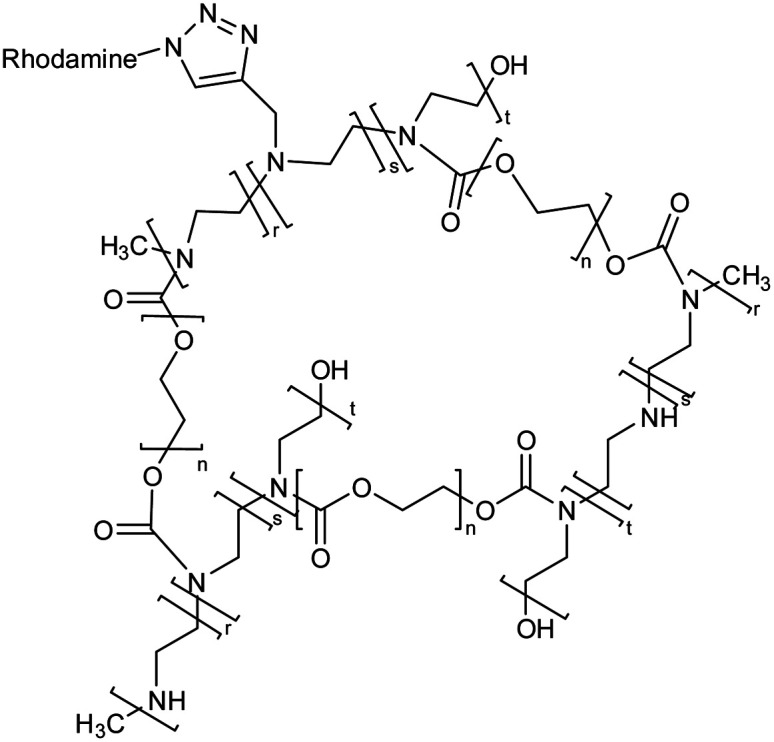
Polyethylene glycol and polyethyleneimine based nanogel |
|
The nanogel loaded with the anti-hepatic stetosis properties bearing polyphenol i.e., hydroxytyrosol (HT) on in vitro administration exhibit the reduction of intracellular triglyceride levels |
Potential pharmacological treatment of non-alcoholic fatty liver disease (NAFLD) |
135
|
| 11 |
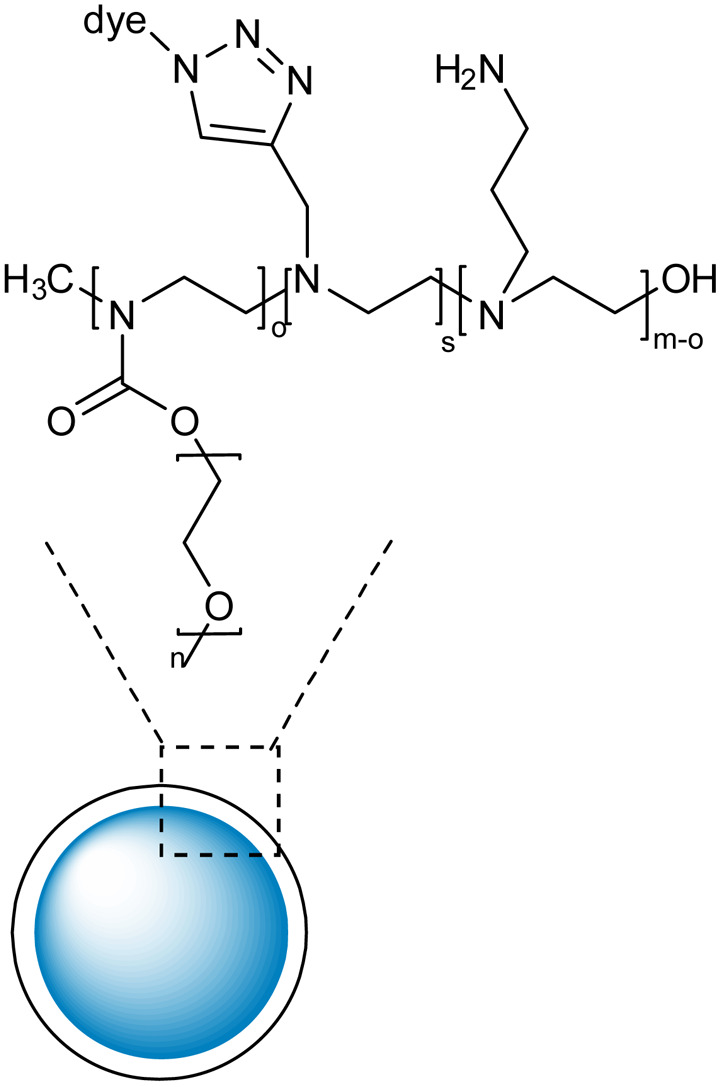
Polyethylene glycol and polyethyleneimine-based nanogels with high colloidal stability and biocompatibility |
|
RhB and Cy5 fluorescent dyes bearing azide groups were used for the synthesis of the nanogels and utilizing the unreacted amines in the PEI chains, the primary amines linkers were grafted around the nanogel surface |
Drug carrier for rolipram to reduce astrogliosis |
136
|
| 12 |
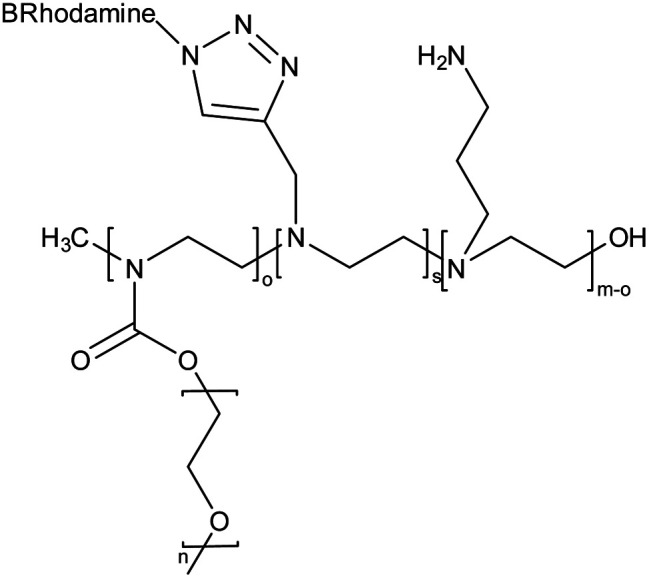
Amine and pyridine-functionalized PEG-PEI based nanogel |
|
The nanogel was synthesized via emulsification-evaporation method and a microscopic molecular examination of the surface features of these systems and an evaluation of their cytocompatibility were also performed |
Efficient drug delivery devices with prolonged sustained release |
137
|
| 13 |
Polyethylene glycol (PEG) and polyethylene-imine (PEI) based nanogel coated with primary amines |
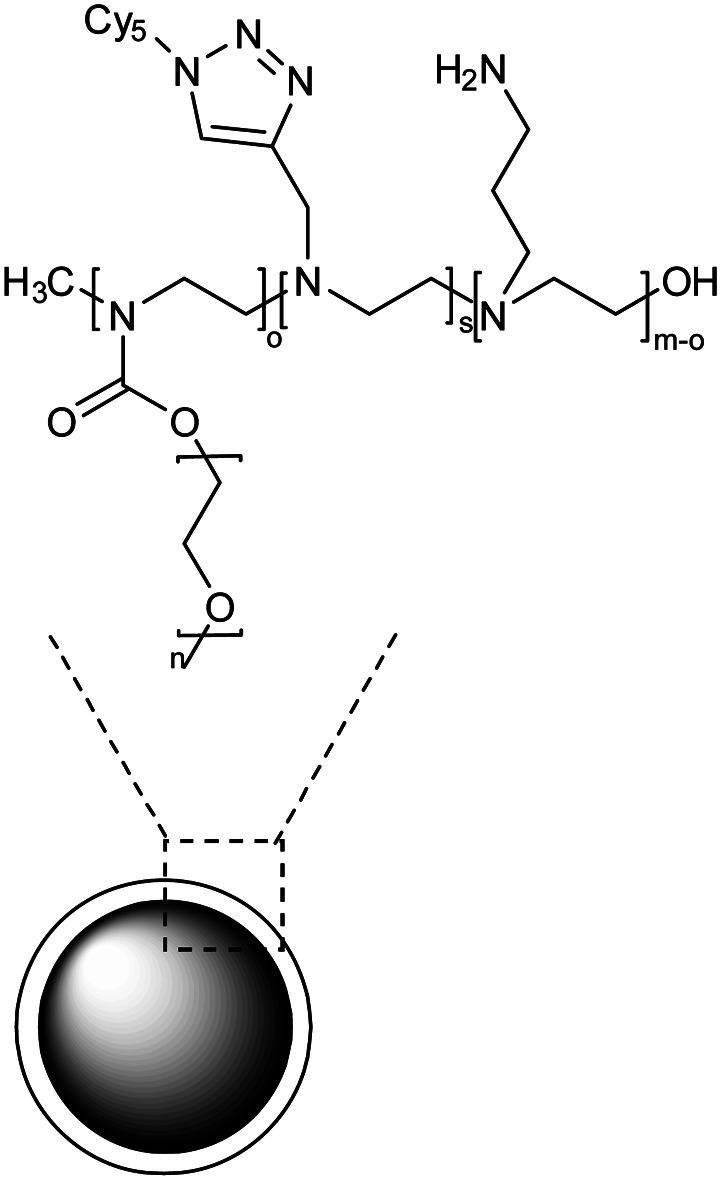
|
The nanogel was synthesized by emulsification-evaporation method and experimental analysis indicated that activated astrocytes were more likely to be targeted by NG in vitro than microglia or neurons |
Targeted delivery of rolipram to quench the pro-inflammatory effects mediated by astrocyte activation |
138
|
| 14 |
Poly(ε-caprolactone)-b-poly(glycidyl methacrylate) (PCL-b-PGMA) block copolymers based nanogel |
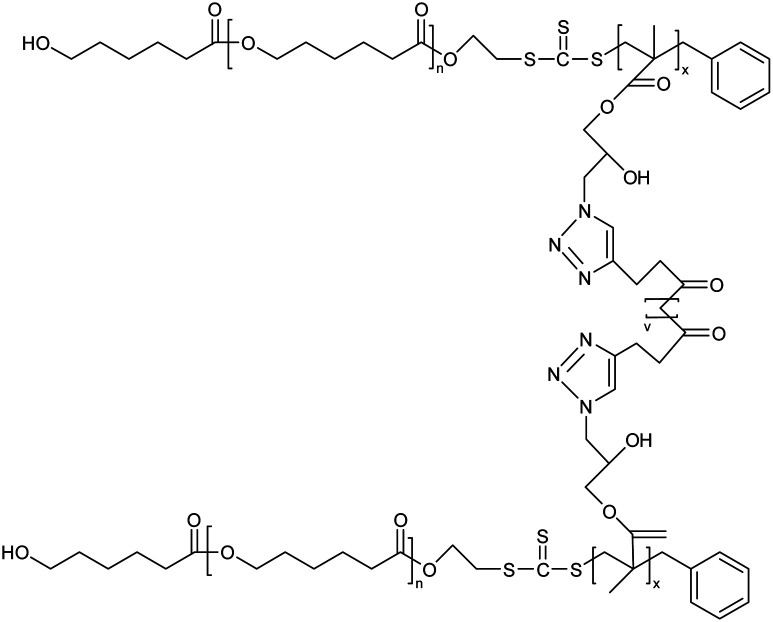
|
A combination of ring-opening polymerization and reversible addition–fragmentation chain transfer polymerization was implied for the synthesis of copolymers for nanogel production |
Potential carrier for low molecular weight drugs |
124
|
| 15 |
Poly-amino acid-based nanogel |
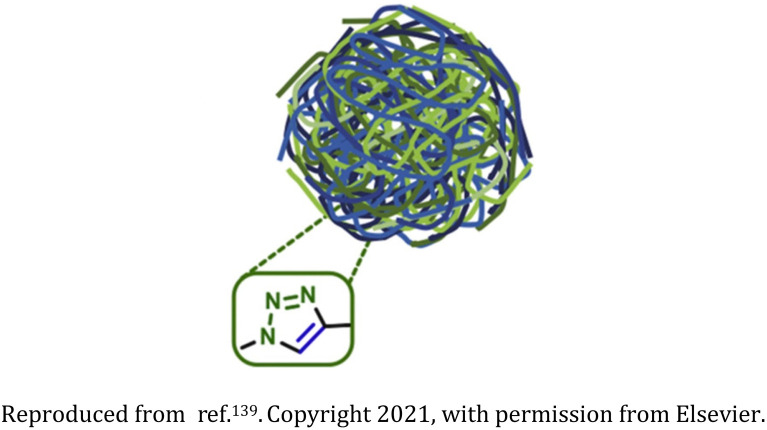
|
Biodegradable and biocompatible cross-linked nanogels synthesized from polyglutamic acid ∼100 nm in size |
Hydrophilic drug carriers for the chemotherapeutic agent doxorubicin for triple-negative breast cancer treatment |
139
|
| 16 |
Anisotropic gold nanoparticles incorporated-polymeric thermoresponsive nanogel |
|
Nanogel was thermoresponsive in nature and synthesized via click chemistry and thermo-nanoprecipitation with gold nanoparticles as the crosslinking agent |
High NIR light-to-heat conversion efficiency for photothermal therapy |
140
|
| 17 |
Photoresponsive oligonucleotide-based nanogel |
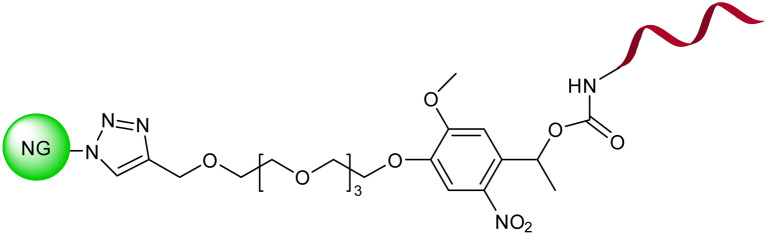
|
The photoirradiation of nanogel led to hyperchromic shift at 260 nm and a simultaneous redshift of the absorption in the 350–400 nm region |
Control of protein release via managing the dissociation of a protein's DNA complex |
141
|
| 18 |
Thiacalix[4]arene functionalized chitosan-based nanogel (CS-g-TC4A) |
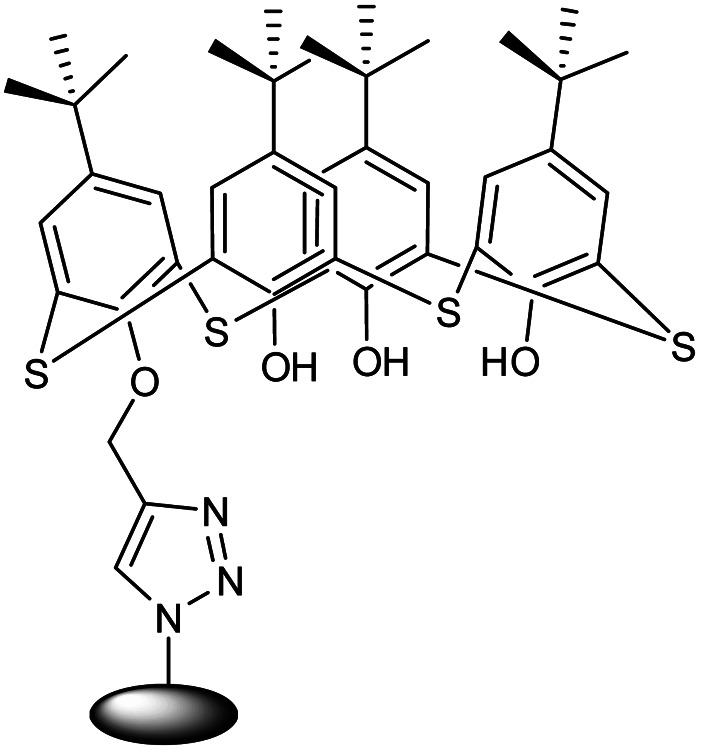
|
The unique multifunctional nano adsorbent nanogel was an efficient sorption agent with controlled super-paramagnetic and sorption capabilities |
The synthesized CS-g-TC4A nanogel having a triazole ring was used as an effective adsorbent for metal ions |
142
|
| 19 |
Hyaluronan and riboflavin derivatives based nanohydrogel |
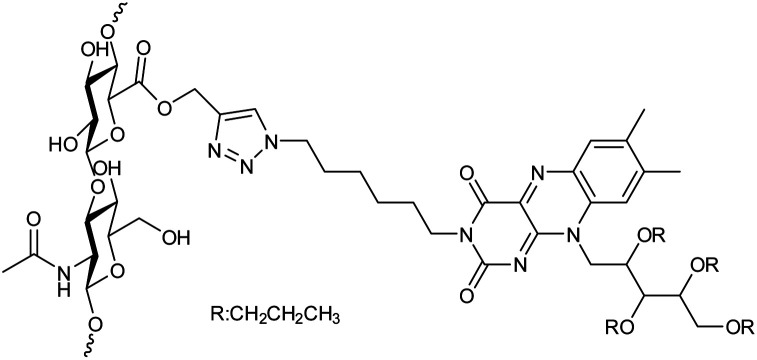
|
The nanohydrogels formulation could be freeze-dried when dextrose was added as a cryoprotectant since they were extremely stable in water solutions |
Drug carriers for the delivery of hydrophobic drugs |
143
|
| 20 |
Thermoresponsive poly(N-vinylcaprolactam) nanogels functionalized with glucose and maltose |
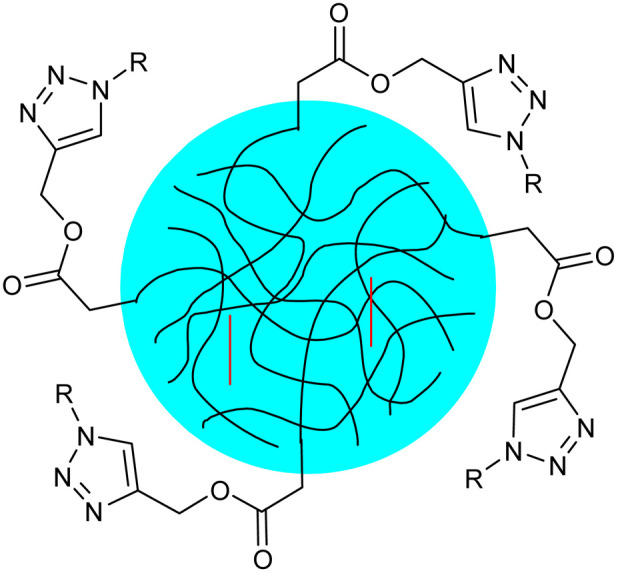
|
The nanogels were synthesized via semibatch precipitation polymerization which shrank upon heating and was equipped with a thermoresponsive structure and surface functionalities that allow for biorelevant interactions |
Enhanced affinity for biomolecules due to the presence of carbohydrates results in greater potential for therapeutic uses as drug-delivery agents |
144
|
| 21 |
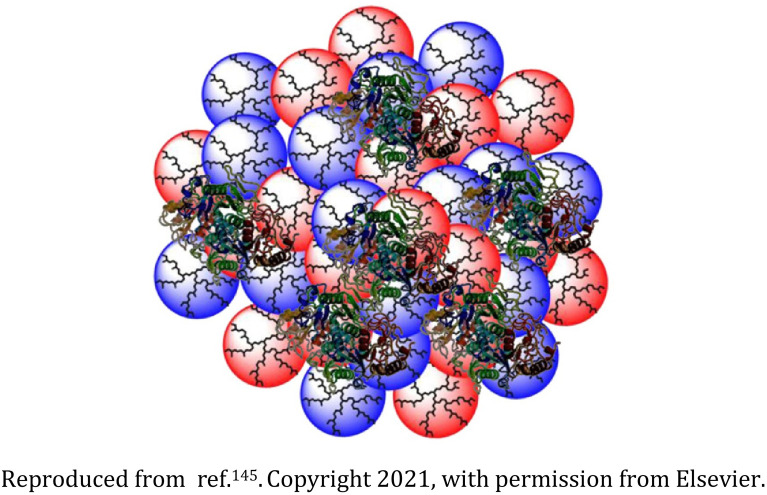
Biodegradable dendritic polyglycerol nanogel |
|
The dendritic polyglycerol nanogels were synthesized by inverse nanoprecipitation wherein the introduction of benzacetal bonds induced the biodegradability |
Effective protein encapsulation and release among other biomacromolecules |
145
|
| 22 |
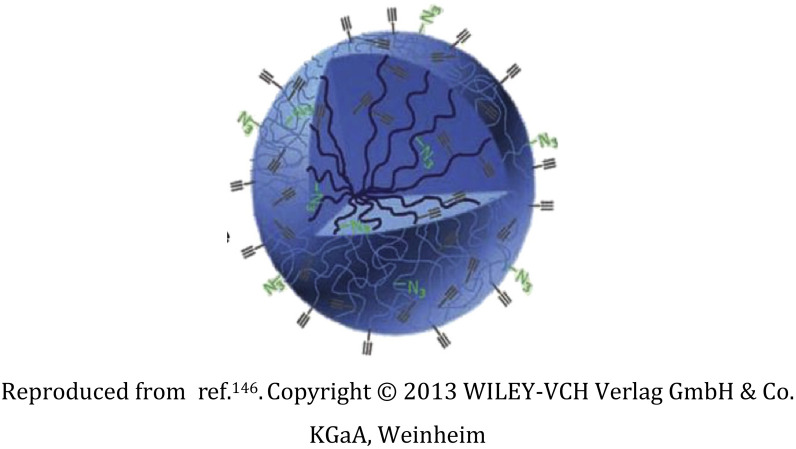
Bisphosphonate ligand-functionalized dextran nanogel |
|
Controlled functionalization of the nanogel with bisphosphonate ligand through astoichiometric click-chemistry in-emulsion method |
Hepatic avoidance, binding to the bone on the inside of marrow cavities, and anti-osteoporotic actions |
146
|
| 23 |
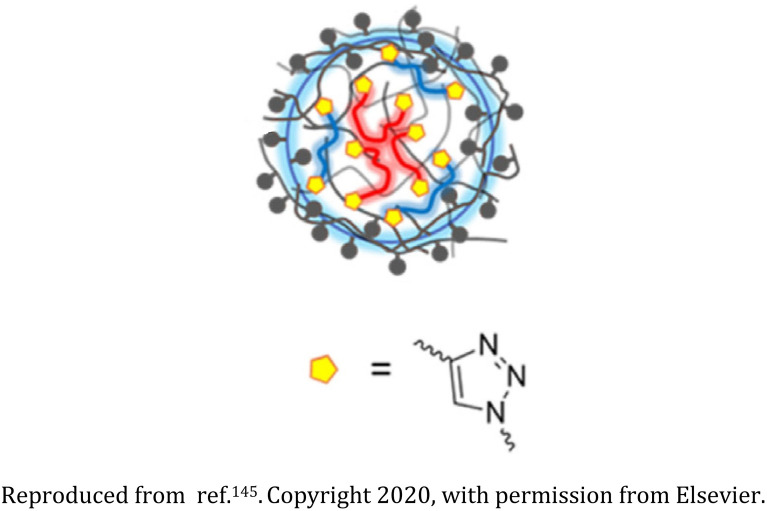
Alkynopoly(acrylic acid), diazido-poly(ethylene glycol), and diazido-poly(butylene succinate) based nanogel |
|
Amphiphilicity of the nanogel is controlled via variations in the hydrophobic diazido-poly(butylene succinate) and the hydrophilic diazido-poly(ethylene glycol) |
Interaction with multi-walled carbon nanotubes (MWCNTs), thereby acting as a potential dispersant of MWCNTs in hydrophilic media |
147
|
| 24 |
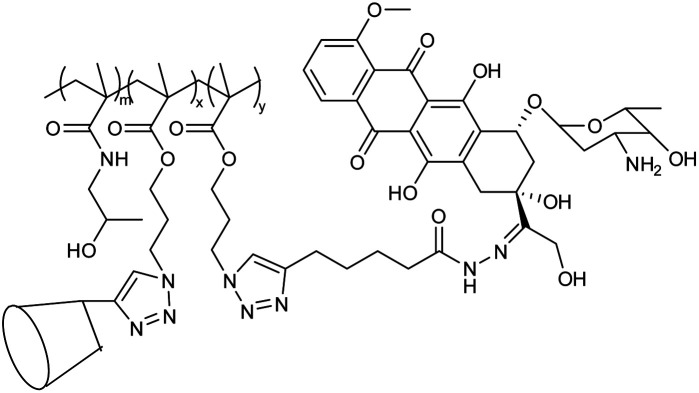
Nanogel based on (adamantly (AD)-benzoic imine-conjugated poly [poly(ethylene glycol) monomethyl ether methacrylate]-co-poly(2-hydroxyethyl methacrylate) (PPEGMA-co-PHEMA-AD) as well as doxorubicin (DOX)-hydrazone and b-cyclodextrin(b-CD) |
|
The polymeric nanogels were synthesized via host–guest interactions, for dual pH-triggered multistage drug delivery, and nanogels were reorganized into smaller nanoparticles in response to tumor acidity |
Deep penetration of the drug at the tumor site, the release of doxorubicin in endosomal or lysosomal acidity (pH ∼5) |
148
|
| 25 |
A pH and reduction dual-sensitive prodrug nanogel |
|
The nanogel displayed excellent size stability against large-volume dilution, high salt concentration, and prolonged incubation in phosphate-buffered saline |
Release doxorubicin in the intracellular acidic and reducible environment after being successfully absorbed by cancer cells |
149
|

























