Abstract
Au-Fe3O4 multifunctional nanoparticles (NPs) were synthesized and integrated with lateral flow immunoassay (LFIA) for dual-mode detection of Salmonella typhimurium. The Au-Fe3O4 NPs not only combined excellent local surface plasmon resonance characteristics and superparamagnetic properties, but also exhibited good photothermal effect. In the detection, antibody-conjugated Au-Fe3O4 NPs first captured S. typhimurium from complex matrix, which was then loaded on the LFIA strip and trapped by the T-line. By observing the color bands with the naked eyes, qualitative detection was performed free of instrument. By measuring the photothermal signal, quantification was achieved with a portable infrared thermal camera. The introduction of magnetic separation achieved the enrichment and purification of target bacteria, thus enhancing the detection sensitivity and reducing interference. This dual-mode LFIA achieved a visual detection limit of 5 × 105 CFU/mL and a photothermal detection limit of 5 × 104 CFU/mL. Compared with traditional Au-based LFIA, this dual-mode LFIA increased the detection sensitivity by 2 orders of magnitude and could be directly applied to unprocessed milk sample. Besides, this dual-mode LFIA showed good reproducibility and specificity. The intra-assay and inter-assay variation coefficients were 3.0% and 7.9%, and with this dual-mode LFIA, other bacteria hardly produced distinguishable signals. Thus, the Au-Fe3O4 NPs-based LFIA has potential to increase the efficiency of pandemic prevention and control.
Graphical Abstract
Au-Fe3O4 nanoparticle proved to be a promising alternative reporter for LFIA, achieving multifunctions: target purification, target enrichment, visual qualitation, and instrumental quantification, which improved the limitations of traditional LFIA.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00604-023-05645-x.
Keywords: Magnetic separation, Bacteria, LFIA strip
Introduction
Food safety problem continues to present a major threat to human health and social security. According to the World Health Organization, foodborne diseases cause ca. 600 million to fall ill, 420,000 to die, and hundreds of billions of US$ lost years, which severely threaten human health and impede socioeconomic development [1]. To effectively reduce the above losses, early and reliable detection plays a key role [2, 3]. Salmonella, as one of the most common foodborne pathogens, affects millions of people annually, sometimes with severe and fatal outcomes, and thus has always been an important monitoring target for food safety [1, 4, 5]. Currently, the main methods used in practice are culture-based assays, polymerase chain reaction (PCR) analyses, and enzyme-linked immunosorbent assay (ELISA). Culture-based assays are the gold standard method owing to their high accuracy but limited by time-consuming and labor-intensive manipulation. PCR has high sensitivity but requires sophisticated instruments and skilled technical staffs, restricting its applications. ELISA simplifies the detection procedure to a certain degree, yet it still suffers from the limitations like relatively low sensitivity and time consumption [6–9]. Thus, these methods cannot be applied to on-site detection or large-scale screening, which seriously restricts the efficiency of pandemic prevention and control.
Lateral flow immunoassay (LFIA) has shown great promise in on-site instant diagnosis, due to its enormous advantages including high speed, low cost, portability, convenience, free of instrument or skilled staff [10–12]. The most successful application of LFIA is the pregnancy test strips, which change hospital-dependent detection to rapid home self-testing, thereby highly improving diagnosis efficiency and reducing costs [13, 14]. However, applying traditional LFIA to bacteria detection faces three main bottleneck problems. One is the low sensitivity which may result in missed detection. The second is that it is a yes-or-no diagnosis which cannot provide accurate quantitative information for guiding therapy. The third is that it cannot be directly applied to complex matrix which must be diluted before flowing on the strips, and this seriously limits its practical application due to the fact that many pathogenic bacteria usually exist in complex biological samples at very low concentration [10, 15–17]. The key reason that induces the above drawbacks is that traditional LFIA mainly uses gold nanoparticles (NPs) as reporters.
In recent decades, researchers have been trying to develop various nanomaterials as new reporters to replace gold NPs, such as dyed beads, fluorescent NPs, surface-enhanced Raman scattering-active nanomaterials, and carbon nanomaterials, which have increased the sensitivity and realized quantitative detection [18–21]. Nevertheless, with these reporters, dilution is still necessary for complex samples, which may lower the sensitivity in practical detection. Magnetic NPs may be promising alternative reporters to solve this problem. Magnetic NPs can effectively capture, separate, and concentrate target bacteria from a large volume of sample into a small volume of buffer under an external magnetic field, thereby realizing purification and enrichment [22–25]. Some works have confirmed the superiority of magnetic reporters in the LFIA. For example, Hwang et al. coated Au NPs on Fe3O4 nanoclusters, with which sensitive detection of Salmonella in milk was achieved [26]. As other examples, Hu et al. and Wang et al., respectively, prepared fluorescent-magnetic nanocomposites by repeated assembling methods and developed multimodal LFIAs for Salmonella and S. pneumoniae detection in milk, serum, or whole blood samples [27, 28]. The above magnetic-colorimetric reporters-based LFIA exhibited obvious advantages over conventional LFIA, yet the preparation of the reporters generally involved more than two kinds of nanoparticles and their assembly, which were complicated and time/labor-consuming.
In our previous work, we proposed a universal seed-mediated growth method to directly synthesize hydrophilic coinage metal/iron oxide (M-Fe3O4) heterodimer NPs, such as Ag-Fe3O4, Pd-Fe3O4, and AgPt-Fe3O4 [29–32]. These M-Fe3O4 NPs not only combined multiple functions, but also showed improved bio-modifiability and water compatibility with good tunability. Herein, based on our previous work, we directly synthesized Au-Fe3O4 heterodimer NPs, avoiding the assembly of two kinds of NPs, which was much more convenient and time-saving. The Au-Fe3O4 NPs integrated the local surface plasmon resonance (LSPR) characteristics of Au and the superparamagnetic properties of Fe3O4, which were used as new reporters in LFIA for Salmonella typhimurium detection. Au part provided favorable colorimetric signal due to its excellent LSPR characteristics. Fe3O4 part enabled magnetic separation and enrichment of the targets, improving the detection sensitivity and applicability in complex matrix. Besides, the Au-Fe3O4 NPs exhibited good photothermal effect due to the combination of Au and Fe3O4 [33–35]. By measuring the photothermal signal with a portable infrared thermal camera, accurate quantification of S. typhimurium was achieved. Thus, as new reporters, Au-Fe3O4 possessed multifunctions: target purification, target enrichment, and dual-mode detection (visual qualitation and instrumental quantification), which improved the limitations of traditional LFIA and showed great application potential in practice.
Experimental section
Reagents and instruments
Tetramethylammonium hydroxide (TMAH), Tween 20, polyvinylpyrrolidone (PVP, MW = 10,000), trisodium citrate (TSC), oleic acid, chloroauric acid (HAuCl4), 1-octadecene, and iron pentacarbonyl were purchased from Macklin Biochemical Co., Ltd. HS-PEG-COOH (MW 2000) was bought from Shanghai ToYong Bio-Tech. Co., Ltd. Mouse anti-S. typhimurium monoclonal antibody was obtained from Abcam. N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), morpholino ethanesulfonic acid (MES), goat anti-mouse antibody, N-hydroxysuccinimide (NHS), acridine orange hemi (AO), and bovine serum albumin (BSA) were bought from Sigma-Aldrich. Heat-inactive Escherichia coli, Listeria monocytogenes, S. typhimurium, and Staphylococcus aureus were supplied by Army Medical University. Sample pad, conjugate pad, absorbent pad, nitrocellulose (NC) membrane, and PVC substrate were purchased from Joey-biotech Co., Ltd. Milk was bought from Nei Monggol Yili Industrial Group Co., Ltd. Ultrapure water was produced by a Millipore Milli-Q system.
Transmission electron microscopy (TEM) images were acquired by a microscope (JEOL, JEM 1400). High-resolution (HR) TEM and energy-dispersive X-ray (EDX) elemental mapping were obtained using a microscope (FEI, Tecnai F30). Absorption spectra were recorded with a spectrophotometer (Shimadzu, UV-2450). The test strips were sprayed by HGS510 (AUTOKUN) and divided by HGS210 induction cutting machine (AUTOKUN). Hydrodynamic diameters and zeta potentials were measured by a Zetasizer Nano ZS instrument (Malvern). Photothermal signals were collected by an infrared thermal camera (FOTRIC 226 s) mounted with an 808 laser (FU808AD2000-F34). Magnetic separation was carried out with a magnet (Invitrogen, 12320D). The photographs were taken using iPhone11 (Apple).
Construction of anti-S. typhimurium antibody-modified Au-Fe3O4 NPs
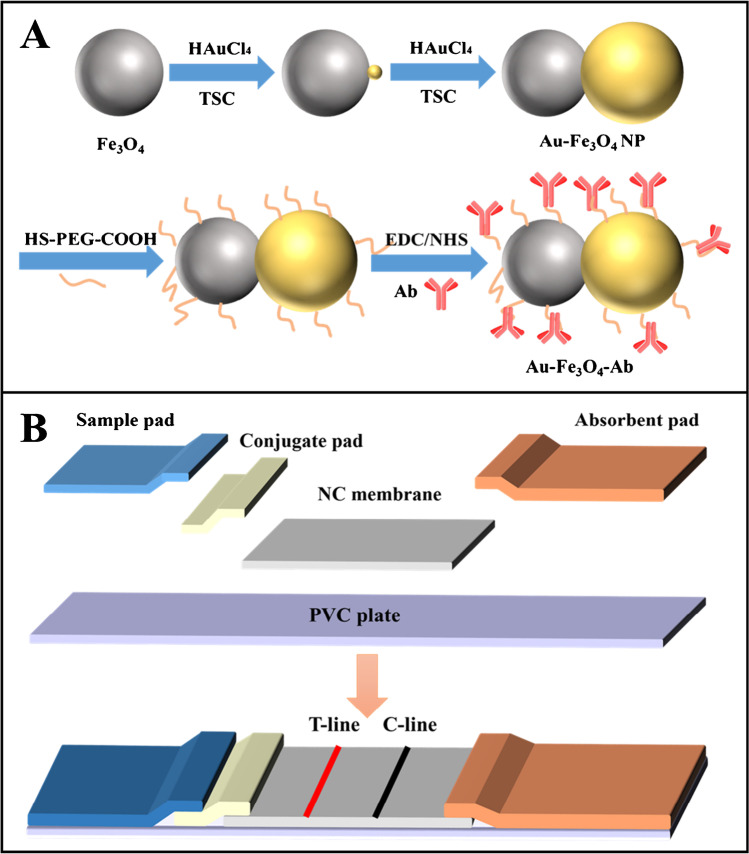
Au-Fe3O4 heterodimer NPs were first prepared by seed-mediated approach referring to our previous work with slight modification [29]. Au3+ was reduced by Fe3O4, producing a gold seed on each Fe3O4 NP, which further grew by repeatedly adding TSC and Au precursor. The detail procedure was presented in the Supporting Information (S.1). Then, the biomodification of Au-Fe3O4 NPs was performed as follows.
Ten milliliters of Au-Fe3O4 NPs was added with 0.01 g of HS-PEG-COOH and shook for 1 h at 37 °C. Then, the above Au-Fe3O4 NPs were washed 3 times with 100 mM MES buffer (pH = 5.0) containing 0.05% Tween 20 and dispersed in 10 mL of MES buffer containing 2.5 mM NHS and 5 mM EDC. After 0.5 h reaction, the Au-Fe3O4 NPs were washed with PBS and dispersed in pH 7.2 PBS (0.01 M, 10 mL) to react with anti-S. typhimurium antibody (10 µg) for about 4 h. Finally, the obtained antibody-functionalized Au-Fe3O4 (Au-Fe3O4-Ab) NPs were washed 3 times by PBS, which were then blocked by 2% BSA, dispersed in 1 mL of PBS, and stored at 4 ℃. The complete preparation and modification process were illustrated in Scheme 1A.
Scheme 1.
A Schematic diagram for the preparation and modification of Au-Fe3O4 NPs. B Schematic diagram for the fabrication of LFIA strip
Fabrication of LFIA strips
As illustrated in Scheme 1B, the test strip was constituted with sample pad, conjugate pad, NC membrane, and absorbent pad pasted onto the PVC plate. The overlap widths were about 2 mm between NC membrane and conjugate pad, 5 mm between NC membrane and absorbent pad, and 5 mm between conjugate pad and sample pad. Mouse anti-S. typhimurium antibody (1.0 mg/mL) was sprayed at the T-line at a rate of 1.0 µL/cm. Goat anti-mouse antibody (1.0 mg/mL) was sprayed at the C-line at a rate of 1.0 µL/cm. Then, the strips were dried for 2 h at 37 °C, which were then cut into 3 mm width and kept in sealed plastic bag.
Detection of S. typhimurium with the dual-mode LFIA
In a typical test, 40 µL of Au-Fe3O4-Ab was added to 600 µL of test samples and incubated at 37 °C with gentle shaking. Then, the mixture was separated by a magnet and resuspended with a solution containing 70 µL of pH 7.2 PBS, 10 µL of Au-Fe3O4-Ab, and 20 µL of running solution (2.0% BSA and 0.25% Tween 20 in pH 7.2 PBS). The above solution was transferred to a microtiter plate and the sample pad of the LFIA strip was immersed in it. All the liquid was absorbed and migrated along the strip. After 15 min, by observing T-line color with the naked eyes, qualitative detection was achieved. Meanwhile, the photothermal signal of the T-line was measured for quantitative detection, which was defined as follows.
where ΔT1 was the temperature variation of the tested strips before and after irradiation and ΔT0 was the temperature variation of the unused strips.
Detection of S. typhimurium in simulated complex samples
The whole milk was first tested by PCR methods to certify that they were not contaminated by S. typhimurium as our previous work [36]. Then, the milk was mixed with S. typhimurium suspension in 0.1 M pH 7.2 PBS (9:1 by volume) to simulate complex samples. For detection, Au-Fe3O4-Ab was added to incubate with the simulated samples. After the reaction, separation, and immunochromatography with the dual-mode strip as described in the previous section, naked-eye observing and photothermal signal measuring were performed. Blank milk samples were treated the same except no bacterium was added. Meanwhile, PBS samples with the same bacteria concentrations were detected for control.
Results and discussion
Principle of the dual-mode LFIA for detecting S. typhimurium
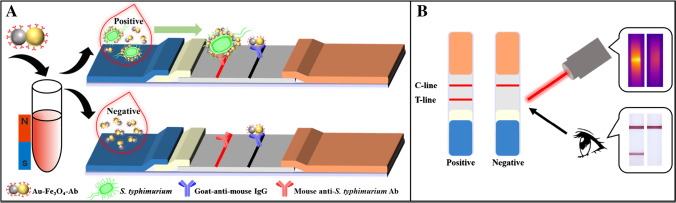
As illustrated by Fig. 1A, Au-Fe3O4-Ab was first added to the samples to capture and separate S. typhimurium. This step achieved enrichment and purification of target bacteria, which would greatly facilitate enhancing detection signal and reducing interference. Then, the obtained Au-Fe3O4-Ab-S. typhimurium complexes were loaded on the LFIA strip, which were captured by the anti-S. typhimurium antibody on the T-line, inducing a colored band. Free Au-Fe3O4-Ab migrated further, which were captured by the anti-mouse antibody on the C-line to produce a quality control band. For negative samples without S. typhimurium, Au-Fe3O4-Ab would flow past the T-line without interaction and only react with the C-line. Thus, as illustrated in Fig. 1B, positive samples produced both colored C-line and T-line, and negative samples only produced colored C-line. While for the ones whose C-line did not show color, indicating the antibody had lost bioactivity, the results were invalid. By observing the color bands with the naked eyes, qualitative detection was performed free of instrument. Meanwhile, by measuring the photothermal signal of the T-line with a portable infrared thermal camera, quantification was achieved. Figure 1B also shows the typical positive and negative results from visual and thermal camera observation. It could be seen that the positive one showed two clear colored bands, and its T-line produced an obvious high temperature zone. While for the negative one, only the C-line produced color, and the temperature of its T-line hardly rose.
Fig. 1.
A Schematic diagram for S. typhimurium detection with the dual-mode LFIA. B Interpretation of the detection results and the typical positive and negative results from visual and infrared thermal camera observation
Characterization of Au-Fe3O4 and Au-Fe3O4-Ab NPs
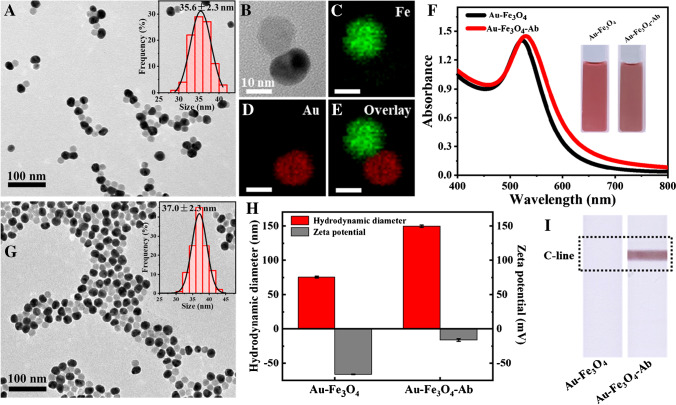
As shown in Fig. 2A-B, Au-Fe3O4 NPs possessed good dispersion with an average size of 35.6 ± 2.3 nm (200 particles were counted for calculation) and mainly exhibited one-to-one dumbbell structure. From the EDX elemental mapping of one single NP (Fig. 2C–E), it could be seen that Fe element and Au element were respectively distributed in the two sections of the heterodimer, which further confirmed its structure. Besides, the Au-Fe3O4 solution showed a wine-red color with a maximum absorbance peak at 522 nm (Fig. 2F).
Fig. 2.
Characterization of Au-Fe3O4 and Au-Fe3O4-Ab NPs. A TEM image and size distribution of Au-Fe3O4 NPs. B HR-TEM image of a single Au-Fe3O4 NP. C–E EDX element mappings of Fe, Au, and the overlay. F UV–Vis spectra of Au-Fe3O4 and Au-Fe3O4-Ab solution and the corresponding photos (inset). G TEM image and size distribution of Au-Fe3O4-Ab NPs. H Hydrodynamic diameters and zeta potentials of Au-Fe3O4 and Au-Fe3O4-Ab. I Photographs of the test strips loaded with Au-Fe3O4 and Au-Fe3O4-Ab NPs
For construction of Au-Fe3O4-Ab, carboxyl groups were first introduced on Au-Fe3O4 NP surface with HS-PEG-COOH based on coordination interaction and then conjugated with the amino groups of anti-S. typhimurium antibody through carbodiimide chemistry. The Au-Fe3O4-Ab remained the optical and morphology properties of Au-Fe3O4 NPs. As shown in Fig. 2F–G, Au-Fe3O4-Ab NPs had good dispersibility with an average size of 37.0 ± 2.3 nm (200 particles were counted for calculation) and exhibited a wine-red color with a maximum absorbance peak at 528 nm, which only slightly changed compared with Au-Fe3O4 NPs. To confirm the conjugation, the hydrodynamic diameter and zeta potential of the NPs before and after functionalization were measured, and the results are shown in Fig. 2H. It could be seen that the hydrodynamic diameter produced an obvious increase from 76 to 150 nm, and the zeta potential changed from − 66 to − 16 mV, which demonstrated the antibody was successfully coupled with the NPs [37–39]. Furthermore, Au-Fe3O4 and Au-Fe3O4-Ab were, respectively, loaded on the LFIA strips, and as shown in Fig. 2I, the strip with Au-Fe3O4-Ab produced a colored C-line, while the strip with Au-Fe3O4 produced no colored line. This demonstrated that the anti-S. typhimurium antibody on the NPs maintained good bioactivity, which could react with the goat anti-mouse antibody on the C-line.
Reliability of the dual-mode LFIA
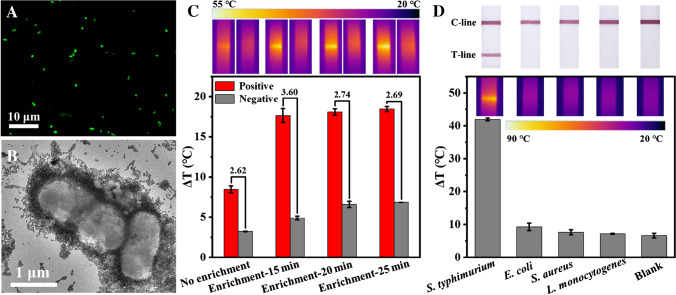
Fluorescence microscopy and TEM analyses were performed to investigate the capture of S. typhimurium with Au-Fe3O4-Ab. From Fig. 3A, it can be seen that many green fluorescence dots, indicating AO-stained bacteria, could be found after the capture of S. typhimurium by Au-Fe3O4-Ab. While using Au-Fe3O4 to treat S. typhimurium, no bacterium was observed (Fig. S1A). This demonstrated that Au-Fe3O4-Ab could efficiently capture S. typhimurium based on antibody-antigen interaction. TEM image more intuitively showed the combination between S. typhimurium and Au-Fe3O4-Ab. From Fig. 3B, it can be seen that many Au-Fe3O4-Ab NPs bound with the bacterium surfaces. While using Au-Fe3O4 to treat S. typhimurium, few NPs combined with S. typhimurium, and after separation, only Au-Fe3O4 NPs were observed rather than bacteria (Fig. S1B-C). This matched well with the microscopy analyses.
Fig. 3.
A–B Fluorescence microscopic image and TEM image of S. typhimurium captured by Au-Fe3O4-Ab. C Photothermal signals obtained from positive samples (1 × 107 CFU/mL S. typhimurium) and blank samples enriched by different magnetic attraction times. The lower is the histogram of photothermal signals, the upper are the corresponding photothermal images. The numbers above the columns are the signal-to-noise ratios. D Results from detection of different bacteria (5 × 107 CFU/mL). The lower is the photothermal signal histogram and images of the test strips, the upper are the corresponding photographs by phones. Error bars are calculated from three experiments
Then, we explored the effect of magnetic enrichment on detection signal. As shown in Fig. 3C, compared with the samples without enrichment, the enriched ones achieved higher photothermal signals, facilitating enhanced detection sensitivity. Besides, the magnetic attraction time in separation also influenced the detection signal, due to the fact that the attraction time affected the capture efficiency. With the increase of attraction time, the signals from positive samples and negative ones both increased, and 15 min induced the highest signal-to-noise ratio (the ratio of the positive sample signal to the negative sample signal), which was chosen as the optimal attraction time. Other conditions, including irradiation power density, irradiation time, amount of Au-Fe3O4-Ab NPs, and incubation time, were also optimized. Finally, 4 W/cm2, 100 s irradiation, 40 µL of Au-Fe3O4-Ab NPs, and 20-min incubation were selected (Fig. S2-5).
Under optimal conditions, the specificity and reproducibility of the dual-mode LFIA were evaluated. S. typhimurium, L. monocytogenes, S. aureus, and E. coli at the same concentration (5 × 107 CFU/mL) and the blank samples without bacteria were detected by the strips. As shown in Fig. 3D, S. typhimurium samples produced obvious colored T-line and strong photothermal signal, while other bacteria showed similar phenomenon as that of the blank, in which no colored T-line or obvious photothermal signal was found. Meanwhile, we calculated the coefficient of variations of the intra-assay and inter-assay as 3.0% and 7.9%, confirming the good reproducibility.
All the above results demonstrated that this Au-Fe3O4 multifunctional NPs-based LFIA enabled efficient capture of S. typhimurium, and the pre-enrichment enhanced the detection signal, definitely improving the sensitivity. Besides, this LFIA had good specificity and reproducibility, assuring the detection reliability.
Analytical performance of the LFIA
To evaluate the sensitivity and dynamic range, the dual-mode LFIA was used to detect different concentrations of S. typhimurium. As shown in Fig. 4A, for the negative samples, only the C-line generated a colored band, and when the S. typhimurium concentration reached 5 × 105 CFU/mL, the T-line began to display a visible color. As the bacteria concentration increased further, the T-line color became darker, due to the fact that more Au-Fe3O4-Ab-S. typhimurium complexes were accumulated on the test zone. Therefore, by observing the color change of the T-line with the naked eyes, qualitative detection of S. typhimurium was achieved with a limit of 5 × 105 CFU/mL. Meanwhile, the photothermal signals of the strips were measured, which are shown in Fig. 4B. Similar with the visual detection, the photothermal signal increased with the bacteria concentration. According to the lowest distinguishable signal (Sblank + 3SD, where Sblank is the average signal of blank groups, and SD is their standard deviation), the limit of the photothermal detection could achieve 5 × 104 CFU/mL, which was one order of magnitude lower than that of the visual observing. Moreover, a good linear relationship was exhibited at the concentration from 5 × 106 to 1 × 108 CFU/mL with R2 = 0.9989 (Fig. 4C), facilitating accurate quantification of S. typhimurium. Compared with the commercial LFIA [27], this dual-mode LFIA increased the sensitivity by 1–2 orders of magnitude, which might be attributed to the pre-enrichment of the target bacteria.
Fig. 4.
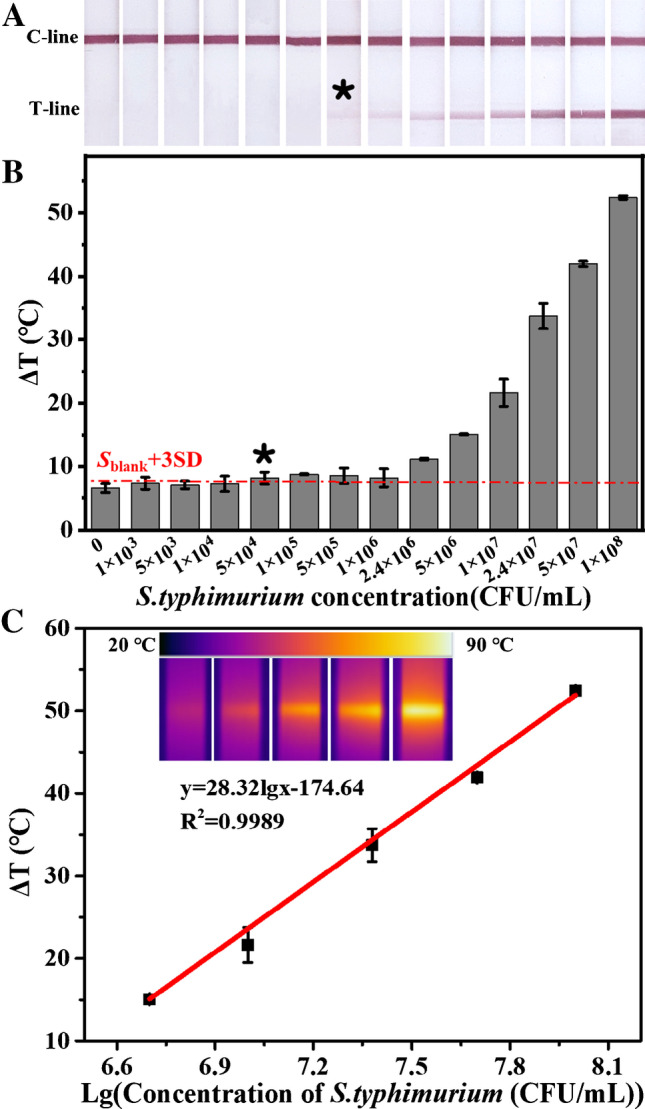
A–B Photographs of the test strips and the corresponding photothermal signals obtained from detection of different concentrations of S. typhimurium. The asterisk represents the detection limit. The red line represents the lowest distinguishable signal by photothermal measuring. C Calibration plot of photothermal signal versus S. typhimurium concentration. The insets are the corresponding photothermal images. Error bars are calculated from three experiments
Application to simulated complex samples
To test the feasibility of the dual-mode LFIA in complex biological matrixes, unprocessed milk spiked with S. typhimurium was used to mimic real samples for detection. As shown in Fig. 5, all the positive milk samples produced two obvious colored bands, and the negative one only produced a colored C-line, which were almost the same with those of the buffer samples. By measuring the photothermal signal, the milk samples induced slightly lower signal than the buffer samples, and the recovery was 72.70 ± 8.28% (Table S1), which was tolerable, due to the complexity of the unprocessed milk, while the commercial LFIA can be only applied to diluted milk, because the unprocessed milk cannot migrate on strips. This dual-mode LFIA’s better analytical performance in complex matrixes was mainly attributed to the pre-enrichment and pre-purification by magnetic separation, which made it possess great potential application in practice.
Fig. 5.
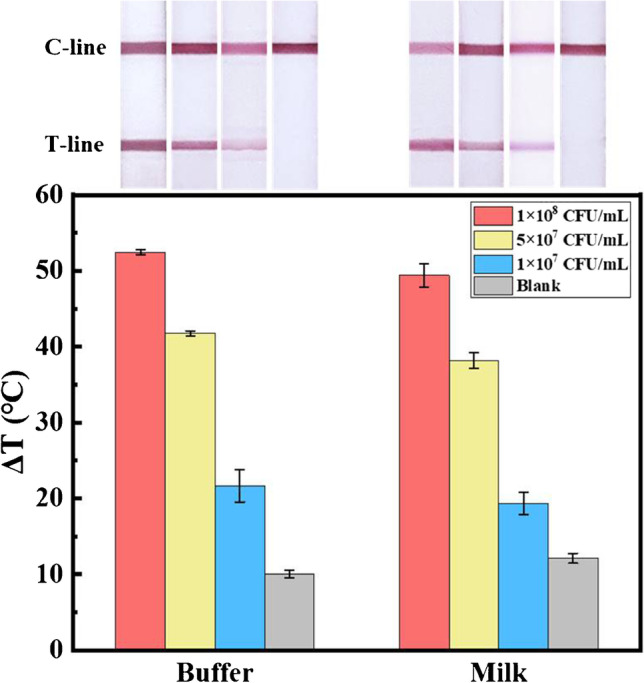
Photothermal signal histograms of the test strips and their corresponding photographs obtained from detecting different concentrations of S. typhimurium in buffer and milk samples. Error bars are calculated from three experiments
Conclusions
In this work, we directly synthesized Au-Fe3O4 multifunctional NPs and integrated them with LFIA as promising alternative reporters for dual-mode detection of S. typhimurium. Table 1 shows the analytical performance comparison between this dual-mode LFIA with other recently reported LFIA. It can be seen that this dual-mode LFIA indeed improved the limitations of traditional LFIA (Au-based LFIA) [40], and it increased the detection sensitivity by 2 orders of magnitude. Moreover, compared with carbon nanoparticles, quantum dots, up-converting materials, etc. [39, 41–44], Au-Fe3O4 NPs enabled enrichment and purification of target bacteria from complex matrix, thus achieving direct application to unprocessed whole milk. Besides, Au-Fe3O4 NPs could provide photothermal signal, facilitating quantitative detection with a portable infrared thermal camera, which was more sensitive than color observing and more cost-effective than fluorescence or magnetic signal determination [27, 45]. However, there is still room for improvement in this method. In the future, we will try to optimize the Fe3O4 size and the Au absorption to obtain faster enrichment and better photothermal signal and then make the manipulation more convenient and achieve more sensitive detection.
Table 1.
An overview on recently reported LFIA for detection of Salmonella
| Materials used | Signal | Detection limit (CFU/mL) | Assay time | Linearity range (CFU/mL) | Complex samples | Ref |
|---|---|---|---|---|---|---|
| Gold nanoparticles | Color | 107 | 30 min | Qualitative | [40] | |
| Gold-coated gold nanoparticles | Color | 104 | 20 min | Qualitative | Diluted fat-reduced milk | [41] |
| Nitrogen-rich carbon nanoparticles | Color | 102 | 20 min | 102–108 | Diluted juice and vegetable salad | [42] |
| Au@Ru nanocomposites | Color | 9.8 × 104 | 10 min | 2.9 × 106–2.9 × 1011 | Diluted drinking water, juice, and milk | [39] |
| Quantum dot nanobeads | Fluorescence | 5 × 103 | 12 min | 5 × 104–107 | Broth processed by centrifugation | [43] |
| Up-converting phosphor | Fluorescence | 104 | 20 min | 104–107 | Food samples cultured for about 14 h | [44] |
| Magnetic nanoparticles | Color | 1.95 × 105 | 30 min | Qualitative | Low fat milk | [45] |
| Fluorescent-magnetic nanospheres | Fluorescence and magnetic signal | 3.5 × 103 | 35 min | 1.88 × 104–1.88 × 107 | Milk, serum, and whole blood | [27] |
| Au-Fe3O4 nanoparticles | Color and photothermal signal | 5 × 104 | 50 min | 5 × 106–1 × 108 | Whole milk | This work |
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was financially supported by the Natural Scientific Foundation of Shandong (ZR2020MB064, ZR2020ZD13, ZR2022JQ07), the Science and Technology Projects of Qingdao (21–1-4-sf-7-nsh), the National Natural Science Foundation of China (No. 21876206), and the Fundamental Research Funds for the Central Universities (No. 22CX03033A).
Data availability
Date will be made available on request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cong-Ying Wen, Email: flcyxt@163.com.
Jing-Bin Zeng, Email: xmuzjb@163.com.
References
- 1.WHO Fact Sheets: Food Safety - the Global View, http://www.who.int/mediacentre/factsheets/fs399/en/, accessed: July 2022
- 2.Shen Y, Zhang Y, Gao ZF, Ye Y, Wu Q, Chen H-Y, Xu J-J. Recent advances in nanotechnology for simultaneous detection of multiple pathogenic bacteria. Nano Today. 2021;38:101121. doi: 10.1016/j.nantod.2021.101121. [DOI] [Google Scholar]
- 3.Furst AL, Francis MB. Impedance-based detection of bacteria. Chem Rev. 2019;119(1):700–726. doi: 10.1021/acs.chemrev.8b00381. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Bai L, Li W, Han H, Fu P, Ma X, Bi Z, Yang X, Zhang X, Zhen S, Deng X, Liu X, Guo Y. Trends of foodborne diseases in China: lessons from laboratory-based surveillance since 2011. Front Med. 2018;12(1):48–57. doi: 10.1007/s11684-017-0608-6. [DOI] [PubMed] [Google Scholar]
- 5.Lu Z, Liu W, Cai Y, Zhao T, Cui M, Zhang H, Du S. Salmonella typhimurium strip based on the photothermal effect and catalytic color overlap of PB@Au nanocomposite. Food Chem. 2022;385:132649. doi: 10.1016/j.foodchem.2022.132649. [DOI] [PubMed] [Google Scholar]
- 6.Varadi L, Luo JL, Hibbs DE, Perry JD, Anderson RJ, Orenga S, Groundwater PW. Methods for the detection and identification of pathogenic bacteria: past, present, and future. Chem Soc Rev. 2017;46(16):4818–4832. doi: 10.1039/c6cs00693k. [DOI] [PubMed] [Google Scholar]
- 7.Chang D, Zakaria S, Esmaeili Samani S, Chang Y, Filipe CDM, Soleymani L, Brennan JD, Liu M, Li Y. Functional nucleic acids for pathogenic bacteria detection. Acc Chem Res. 2021;54(18):3540–3549. doi: 10.1021/acs.accounts.1c00355. [DOI] [PubMed] [Google Scholar]
- 8.Wei L, Wang Z, Feng C, Xianyu Y, Chen Y. Direct transverse relaxation time biosensing strategy for detecting foodborne pathogens through enzyme-mediated sol-gel transition of hydrogels. Anal Chem. 2021;93(17):6613–6619. doi: 10.1021/acs.analchem.0c03968. [DOI] [PubMed] [Google Scholar]
- 9.Qi X, Wang Z, Lu R, Liu J, Li Y, Chen Y. One-step and DNA amplification-free detection of Listeria monocytogenes in ham samples: combining magnetic relaxation switching and DNA hybridization reaction. Food Chem. 2021;338:127837. doi: 10.1016/j.foodchem.2020.127837. [DOI] [PubMed] [Google Scholar]
- 10.Bahadır EB, Sezgintürk MK. Lateral flow assays: principles, designs and labels. TrAC-Trend Anal Chem. 2016;82:286–306. doi: 10.1016/j.trac.2016.06.006. [DOI] [Google Scholar]
- 11.Parolo C, Sena-Torralba A, Bergua JF, Calucho E, Fuentes-Chust C, Hu L, Rivas L, Alvarez-Diduk R, Nguyen EP, Cinti S, Quesada-Gonzalez D, Merkoci A. Tutorial: design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat Protoc. 2020;15(12):3788–3816. doi: 10.1038/s41596-020-0357-x. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Ding L, Huang X, Xiong Y. Tailoring noble metal nanoparticle designs to enable sensitive lateral flow immunoassay. Theranostics. 2022;12(2):574–602. doi: 10.7150/thno.67184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The history of the pregnancy test, https://history.nih.gov/exhibits/thinblueline/timeline.htm#1970, accessed: July 2022
- 14.Bhalla N, Pan Y, Yang Z, Payam AF. Opportunities and challenges for biosensors and nanoscale analytical tools for pandemics: COVID-19. ACS Nano. 2020;14(7):7783–7807. doi: 10.1021/acsnano.0c04421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong Y, Chen Y, Yao L, Zhao D, Zheng L, Liu G, Ye Y, Chen W. Gold nanoparticles based lateral flow immunoassay with largely amplified sensitivity for rapid melamine screening. Microchim Acta. 2016;183(6):1989–1994. doi: 10.1007/s00604-016-1812-9. [DOI] [Google Scholar]
- 16.Sohrabi H, Majidi MR, Khaki P, Jahanban-Esfahlan A, de la Guardia M, Mokhtarzadeh A. State of the art: lateral flow assays toward the point-of-care foodborne pathogenic bacteria detection in food samples. Comp Rev Food Sci Food Saf. 2022;21(2):1868–1912. doi: 10.1111/1541-4337.12913. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Wang Y, Liu Z, Bai M, Wang J, Wang Y. Nanobody-based immunochromatographic biosensor for colorimetric and photothermal dual-mode detection of foodborne pathogens. Sensors and Actuators B: Chemical. 2022;369:132371. doi: 10.1016/j.snb.2022.132371. [DOI] [Google Scholar]
- 18.Fan LZ, Yang J, Wu JB, Li FG, Yan WN, Tan F, Zhang M, Draz MS, Han HX, Zhang PF (2022) Deeply-dyed nanobead system for rapid lateral flow assay testing of drugs at point-of-care. Sensors and Actuators B-Chemical 362. 10.1016/j.snb.2022.131829
- 19.Zheng P, Peng T, Wang J, Zhang J, Wang Z, Zhang Y, Ren Z, Wang S, Jiang H. Fluorescent lateral flow immunoassay based on gold nanocluster for detection of pyrrolizidine alkaloids. Microchim Acta. 2021;188(1):11. doi: 10.1007/s00604-020-04672-2. [DOI] [PubMed] [Google Scholar]
- 20.Liang P, Guo Q, Zhao T, Wen CY, Tian Z, Shang Y, Xing J, Jiang Y, Zeng J. Ag nanoparticles with ultrathin Au shell-based lateral flow immunoassay for colorimetric and SERS dual-mode detection of SARS-CoV-2 IgG. Anal Chem. 2022;94(23):8466–8473. doi: 10.1021/acs.analchem.2c01286. [DOI] [PubMed] [Google Scholar]
- 21.Qiu W, Baryeh K, Takalkar S, Chen W, Liu G. Carbon nanotube-based lateral flow immunoassay for ultrasensitive detection of proteins: application to the determination of IgG. Microchim Acta. 2019;186(7):436. doi: 10.1007/s00604-019-3508-4. [DOI] [PubMed] [Google Scholar]
- 22.Iranmanesh M, Hulliger J. Magnetic separation: its application in mining, waste purification, medicine, biochemistry and chemistry. Chem Soc Rev. 2017;46(19):5925–5934. doi: 10.1039/c7cs00230k. [DOI] [PubMed] [Google Scholar]
- 23.Xiao D, Lu T, Zeng R, Bi Y. Preparation and highlighted applications of magnetic microparticles and nanoparticles: a review on recent advances. Microchim Acta. 2016;183(10):2655–2675. doi: 10.1007/s00604-016-1928-y. [DOI] [Google Scholar]
- 24.Wang Z, Cai R, Gao Z, Yuan Y, Yue T. Immunomagnetic separation: an effective pretreatment technology for isolation and enrichment in food microorganisms detection. Compr Rev Food Sci Food Saf. 2020;19(6):3802–3824. doi: 10.1111/1541-4337.12656. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Xianyu Y, Zhang Z, Guo A, Li X, Dong Y, Chen Y. Background signal-free magnetic bioassay for food-borne pathogen and residue of veterinary drug via Mn(VII)/Mn(II) interconversion. ACS Sens. 2019;4(10):2771–2777. doi: 10.1021/acssensors.9b01349. [DOI] [PubMed] [Google Scholar]
- 26.Hwang J, Kwon D, Lee S, Jeon S. Detection of Salmonella bacteria in milk using gold-coated magnetic nanoparticle clusters and lateral flow filters. RSC Adv. 2016;6(54):48445–48448. doi: 10.1039/c6ra05446c. [DOI] [Google Scholar]
- 27.Hu J, Jiang YZ, Tang M, Wu LL, Xie HY, Zhang ZL, Pang DW. Colorimetric-fluorescent-magnetic nanosphere-based multimodal assay platform for Salmonella detection. Anal Chem. 2019;91(1):1178–1184. doi: 10.1021/acs.analchem.8b05154. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Shen W, Rong Z, Liu X, Gu B, Xiao R, Wang S. Layer-by-layer assembly of magnetic-core dual quantum dot-shell nanocomposites for fluorescence lateral flow detection of bacteria. Nanoscale. 2020;12(2):795–807. doi: 10.1039/c9nr08509b. [DOI] [PubMed] [Google Scholar]
- 29.Zeng J, Gong M, Wang D, Li M, Xu W, Li Z, Li S, Zhang D, Yan Z, Yin Y. Direct synthesis of water-dispersible magnetic/plasmonic heteronanostructures for multimodality biomedical imaging. Nano Lett. 2019;19(5):3011–3018. doi: 10.1021/acs.nanolett.9b00171. [DOI] [PubMed] [Google Scholar]
- 30.Dong Y, Wen CY, She Y, Zhang Y, Chen Y, Zeng J (2021) Magnetic relaxation switching immunoassay based on hydrogen peroxide-mediated assembly of Ag@Au-Fe3 O4 nanoprobe for detection of aflatoxin B1. Small:e2104596. 10.1002/smll.202104596 [DOI] [PubMed]
- 31.Qiu Z, Duan W, Cao S, Zeng T, Zhao T, Huang J, Lu X, Zeng J. Highly specific colorimetric probe for fluoride by triggering the intrinsic catalytic activity of a AgPt-Fe3O4 hybrid nanozyme encapsulated in SiO2 shells. Environ Sci Technol. 2022;56(3):1713–1723. doi: 10.1021/acs.est.1c06453. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Gong M, Li X, Liu H, Liang P, Cui S, Zhang L, Zhou C, Sun T, Zhang M, Wen C-Y, Zeng J. Au-Fe3O4 heterodimer multifunctional nanoparticles-based platform for ultrasensitive naked-eye detection of Salmonella typhimurium. J Hazard Mater. 2022;436:129140. doi: 10.1016/j.jhazmat.2022.129140. [DOI] [PubMed] [Google Scholar]
- 33.Feng W, Zhou X, Nie W, Chen L, Qiu K, Zhang Y, He C. Au/polypyrrole@Fe3O4 nanocomposites for MR/CT dual-modal imaging guided-photothermal therapy: an in vitro study. ACS Appl Mater Interfaces. 2015;7(7):4354–4367. doi: 10.1021/am508837v. [DOI] [PubMed] [Google Scholar]
- 34.Yang Z, Ding X, Jiang J. Facile synthesis of magnetic–plasmonic nanocomposites as T 1 MRI contrast enhancing and photothermal therapeutic agents. Nano Res. 2016;9(3):787–799. doi: 10.1007/s12274-015-0958-9. [DOI] [Google Scholar]
- 35.Wang S, Hou Y. Photothermal therapy based on magnetic nanoparticles in cancer. J Appl Phys. 2021;130(7):070902. doi: 10.1063/5.0057671. [DOI] [Google Scholar]
- 36.Wen CY, Hu J, Zhang ZL, Tian ZQ, Ou GP, Liao YL, Li Y, Xie M, Sun ZY, Pang DW. One-Step sensitive detection of Salmonella typhimurium by coupling magnetic capture and fluorescence identification with functional nanospheres. Anal Chem. 2013;85(2):1223–1230. doi: 10.1021/ac303204q. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Lin L, Yao P, Zhao W, Hu J, Shi XH, Zhang S, Zhu X, Pang DW, Liu AA. Immunoprofiling of severity and stage of bacterial infectious diseases by ultrabright fluorescent nanosphere-based dyad test strips. Anal Chem. 2022;94(24):8818–8826. doi: 10.1021/acs.analchem.2c02028. [DOI] [PubMed] [Google Scholar]
- 38.Yu X, Xia HS, Sun ZD, Lin Y, Wang K, Yu J, Tang H, Pang DW, Zhang ZL. On-chip dual detection of cancer biomarkers directly in serum based on self-assembled magnetic bead patterns and quantum dots. Biosens Bioelectron. 2013;41:129–136. doi: 10.1016/j.bios.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Ji L, Zhang L, Yang H, Liang S, Pan J, Zou Y, Li S, Li Q, Zhao S. Versatile Au@Ru nanocomposites for the rapid detection of Salmonella typhimurium and photothermal sterilization. J Colloid Interface Sci. 2022;621:489–498. doi: 10.1016/j.jcis.2022.04.028. [DOI] [PubMed] [Google Scholar]
- 40.Liu CC, Yeung CY, Chen PH, Yeh MK, Hou SY. Salmonella detection using 16S ribosomal DNA/RNA probe-gold nanoparticles and lateral flow immunoassay. Food Chem. 2013;141(3):2526–2532. doi: 10.1016/j.foodchem.2013.05.089. [DOI] [PubMed] [Google Scholar]
- 41.Bu T, Huang Q, Yan L, Huang L, Zhang M, Yang Q, Yang B, Wang J, Zhang D. Ultra technically-simple and sensitive detection for Salmonella enteritidis by immunochromatographic assay based on gold growth. Food Control. 2018;84:536–543. doi: 10.1016/j.foodcont.2017.08.036. [DOI] [Google Scholar]
- 42.Wang Z, Yao X, Wang R, Ji Y, Yue T, Sun J, Li T, Wang J, Zhang D. Label-free strip sensor based on surface positively charged nitrogen-rich carbon nanoparticles for rapid detection of Salmonella enteritidis. Biosens Bioelectron. 2019;132:360–367. doi: 10.1016/j.bios.2019.02.061. [DOI] [PubMed] [Google Scholar]
- 43.Hu J, Tang F, Jiang YZ, Liu C. Rapid screening and quantitative detection of Salmonella using a quantum dot nanobead-based biosensor. Analyst. 2020;145(6):2184–2190. doi: 10.1039/d0an00035c. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y, Wang H, Zhang P, Sun C, Wang X, Wang X, Yang R, Wang C, Zhou L. Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel lateral flow assay. Sci Rep. 2016;6:21342. doi: 10.1038/srep21342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duan M-L, Huang Y-M, Wu S-S, Li G-Q, Wang S-Y, Chen M-H, Wang C, Liu D-F, Liu C-W, Lai W-H. Rapid and sensitive detection of Salmonella enteritidis by a pre-concentrated immunochromatographic assay in a large-volume sample system. RSC Adv. 2017;7(87):55141–55147. doi: 10.1039/c7ra11006e. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Date will be made available on request.