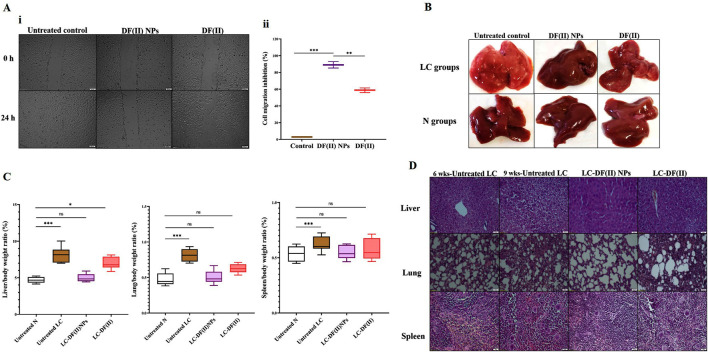
FIGURE 2.
In vitro anti-migration potential and in vivo anti-liver cancer efficacy (morphology, weight, and histochemical assessments) using chemically induced animal model (A) Anti-migration activity as illustrated by (I) microscopic images of migration (at 0 and 24 h) of the untreated and DF(II) complexes-treated HepG2 cells and (II) migration inhibition percentages. Data are shown as mean ± SEM. DF(II) NPs-treated cells were compared to the untreated and DF(II) complexes-treated cells, considering statistically significant at p ≤ 0.05*, ≤0.005**, and ≤0.001***. (B) Macroscopic images of liver morphology of all studied groups (C) Relative liver, lung, and spleen weights to body weight in the untreated and DF(II) complexes-treated liver cancer (LC) groups, compared to healthy (N) group. Data are shown as mean ± SEM. Healthy N group was compared to the untreated and DF(II) complexes-treated LC groups, considering statistically significant at p ≤ 0.05*, ≤0.005**, and ≤0.001*** (C) H&E-staining liver, lung, and spleen tissues of the untreated LC group, at the 6th week of LC induction (showing neoplasia in liver, small section of lung cancer cells without modifications in spleen) and at the 9th of LC induction (declaring a characterized anaplasia (clear cell hepatocellular carcinoma, expanded area of lung cancer, and lymphoid hyperplasia in spleen), and DF(II) complexes-treated LC groups (3 weeks of treatment), disclosing stronger therapeutic efficacy of DF(II) NPs than DF for eradicating LC cells with halting metastasis to lung or spleen.