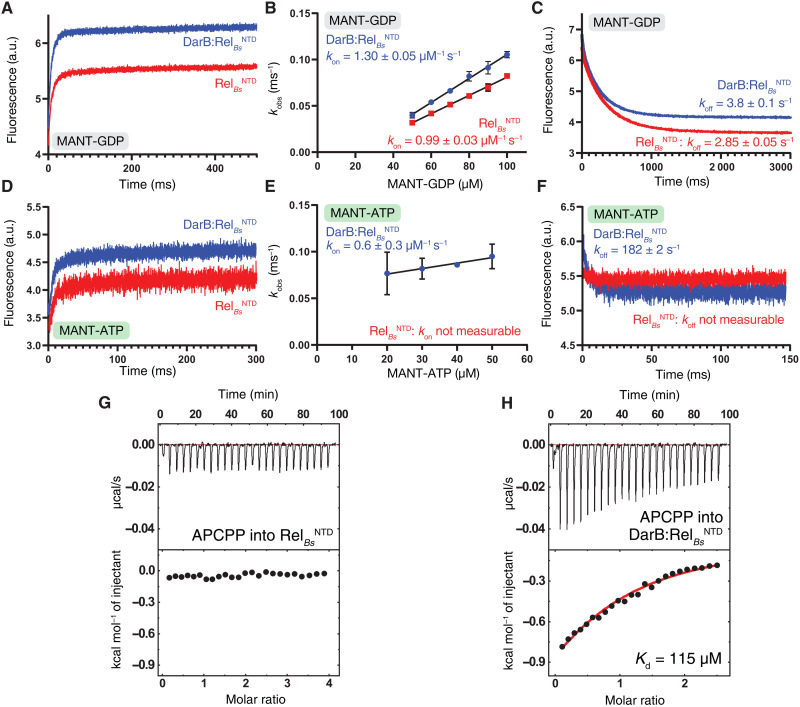
Fig. 5. Kinetics and thermodynamics of nucleotide binding to RelBs in the presence and absence of DarB.
(A) Kinetics of MANT-GDP binding to RelBsNTD (in red) and DarB:RelBsNTD (in blue) monitored by stopped flow. The interaction was measured by FRET excitation of MANT fluorescence upon mixing 10 μM protein with increasing concentrations of MANT-GDP (B). (C) Kinetics of MANT-GDP dissociation from RelBsNTD (in red) and DarB:RelBsNTD (in blue). (D) Kinetics of MANT-ATP binding to RelBsNTD (in red) and DarB:RelBsNTD (in blue). The interaction was measured as in (A) by FRET excitation of MANT-ATP fluorescence upon mixing 10 μM protein with increasing concentrations of MANT-ATP (E). (F) Kinetics of MANT-ATP dissociation from RelBsNTD (in red) and DarB:RelBsNTD (in blue). In both cases, the dissociation was monitored upon rapid mixing with an excess (2 mM) of unlabeled GDP or ATP. Binding of APCPP to RelBsNTD (G) and DarB:RelBsNTD (H) monitored by ITC.