FIG 1.
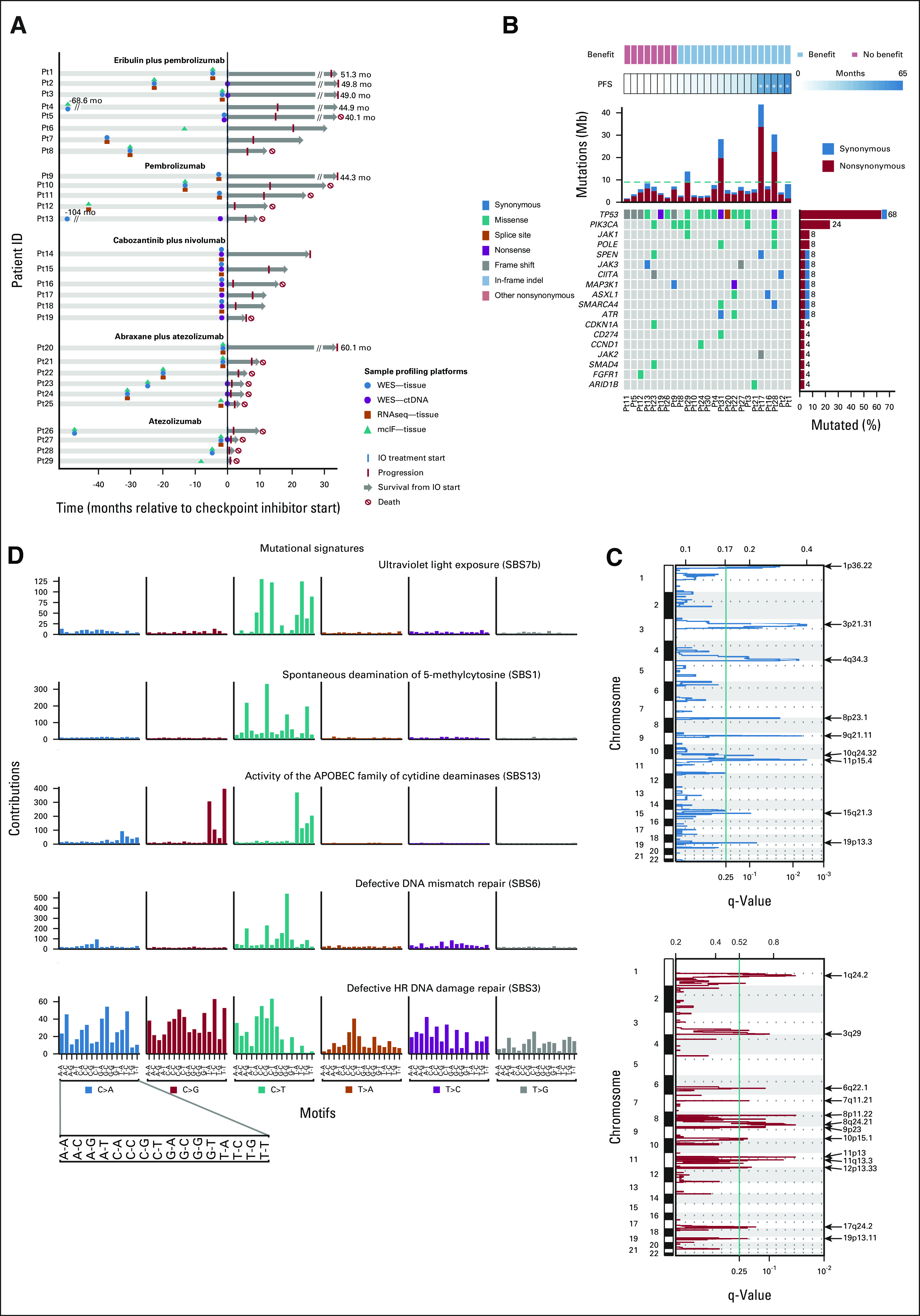
Genomic characteristics of the study population. (A) Summary of clinical history, sample collection, and molecular profiling of patients included in this study. (B) Most frequent mutations observed in 25 tumor samples collected before starting ICI-based therapy. Top tracks, benefit status, and PFS per patient. Patients are sorted by increasing PFS. Five patients with durable responses are marked with an asterisk. Top histogram, mutation rate per sample. Right histogram, frequency of somatic alterations. heatmap, and distribution of synonymous and nonsynonymous mutation events. (C) Recurrent focal deletions (top panel) and amplifications (bottom panel) identified by GISTIC2. (D) Mutational signatures prevalent in the cohort. ctDNA, circulating tumor DNA; HR, homologous recombination; ICI, immune checkpoint inhibitor; mcIF, multicolor immunofluorescence; PFS, progression-free survival; RNASeq, RNA sequencing; SBS, single base substitution; WES, whole-exome sequencing.
