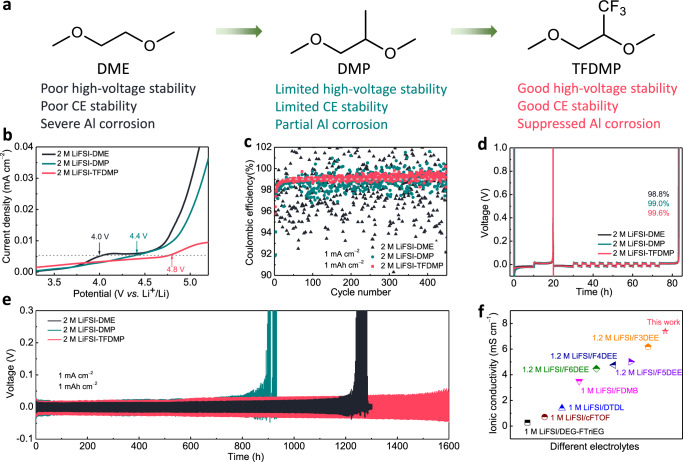
Fig. 1. Schematic of solvent fluorination and electrochemical characterizations of various non-aqueous electrolyte solutions.
a Design of solvent structure and associated properties. b Oxidation stability of electrolytes in Li||Al asymmetric cells tested by linear sweep voltammetry (LSV) at a scan rate of 0.5 mV s–1, the gray dotted line represents the same oxidation current density of three electrolytes. c CE test of Li||Cu asymmetric cells using different electrolytes at 1 mA cm−2 with a cutoff capacity of 1 mAh cm−2. d Average CE test of three electrolytes by Aurbach method40. e Voltage−time profiles of Li||Li symmetric cells with different electrolytes. Testing temperature is 25 ± 1 oC. f Comparisons of bulk ionic conductivities with previously reported electrolytes based on fluorinated-ether solvents22,23,31,44,45, reference articles cited did not report the testing temperature for the ionic conductivity.