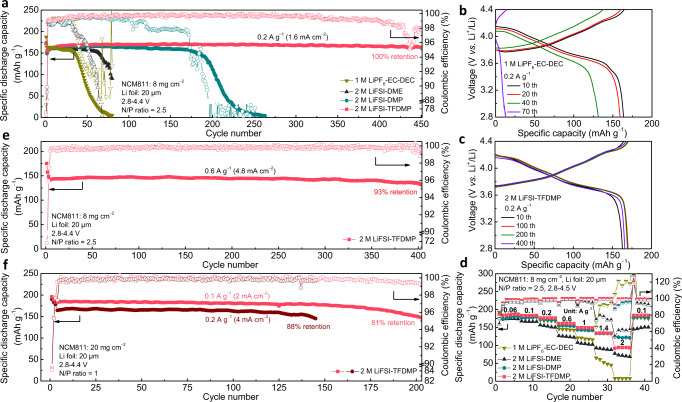
Fig. 4. Electrochemical energy storage performance of Li||NCM811 cells with various electrolyte solutions.
a Cycling test of Li||NCM811 cells with different electrolytes at 0.2 A g–1 (1.6 mA cm−2) after one formation cycle at 0.02 A g–1. b, c The corresponding charge-discharge profiles at different cycles with 1 M LiPF6-EC-DEC (b) and 2 M LiFSI-TFDMP (c). d Rate performance of Li||NCM811 cells with different electrolytes after one formation cycle at 0.02 A g–1 (keeping charge and discharge at the same specific current). e Cycling test of Li||NCM811 cells with 2 M LiFSI-TFDMP electrolyte at 0.6 A g–1 (4.8 mA cm−2) after formation cycles of 0.02 A g–1, 0.2 A g–1, and 0.4 A g–1, one cycle for each. f Performance of high loading Li||NCM811 cells with 2 M LiFSI-TFDMP electrolyte at 0.1 A g–1 (2 mA cm−2) and 0.2 A g–1 (4 mA cm−2) after formation cycles of 0.02 A g–1, 0.06 A g–1, and 0.1 A g–1, one cycle for each. The mass of the specific current and specific capacity referred to the mass of the active material in the positive electrode. All tests were conducted in coin cells at 25 ± 1 °C.