TABLE 1.
Phytochemicals identified in M. oleifera with neuropharmacological effects and their mechanisms of action.
| Compound | Chemical structure | Bioassay methods | Mechanisms of action | References |
|---|---|---|---|---|
| Phenolic acids | ||||
| Gallic acid |
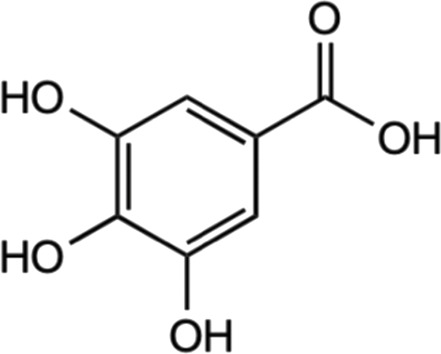
|
Deoxyribose and hydrogen peroxidase assay | Enhanced deoxyribose oxidation and neutralizes free radicals. | Yen et al. (2002) |
| In vitro assay using glioma cell | Dowregulated the PI3K/Akt and rat sarcoma virus (Ras)/MAPK signaling pathways by suppressing coding gene (ADAM17), p-Akt, and p- extracellular signal-regulated kinase (Erk) expression. | Lu et al. (2010) | ||
| In vitro using primary rat cortex neuronal culture | Provide anti-oxidative activities and suppressed regulation of proinflammatory cytokines. | Maya et al. (2018) | ||
| *Glutamate-induced neurotoxicity | ||||
| In vivo male Sprague-Dawley | Mediated with biomarkers of activated astrocytes and microglia, proinflammatory enzymes and cytokines, apoptotic cells. | Liu et al. (2020a) | ||
| *LPS-induced neuroinflammation | ||||
| Chlorogenic acid |
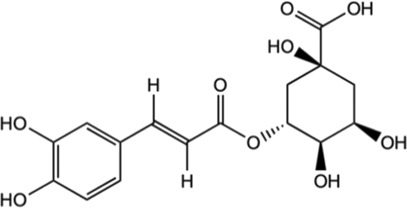
|
In vitro assay using primary cortical neurons | Mediation Nrf2-NF-κB pathways by the activation of Sirt1 and further reduce the apoptosis of brain neurons. | Zheng et al. (2022) |
| In vivo male Sprague Dawley rats | Suppressed hypoxia ischemia-induced proliferation of glia to alleviate the brain injury. | |||
| *Hypoxia-ischemia brain injury | ||||
| Inhibited the expression of TNF-α, IL-1β, and nitric oxide synthase (iNOS) in the brain tissue. | ||||
| Ferulic acid |
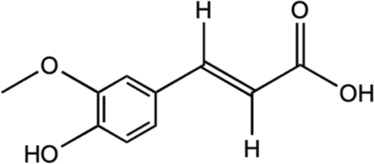
|
In vitro assay using SH-SY5Y neuroblastoma cells | Activated HO-1/Nrf2 system through carbon monoxide and bilirubin production. | Catino et al. (2016) |
| Prevented disruption of cell lines by up-regulating the HO-1/Nrf2 system. | ||||
| Caffeic acid |
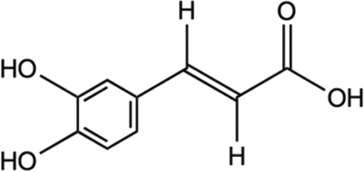
|
In vivo male Swiss albino mice | Supressed production of LPS-induced TNF-α and IL-6. | Mallik et al. (2016) |
| Reducing the levels of malondialdehyde and GSH as well as inhibiting the c-Src/ERK pathway of MAPK activation to alleviate oxidative stress. | ||||
| *LPS-induced neuroinflammation | Facilitated downregulation of NF-κB-dependent pro-inflammatory genes. | |||
| Flavonoids | ||||
| Kaempferol |
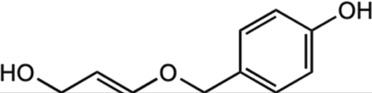
|
In vitro assay using murine microglial BV2 cells | Alleviated LPS-induced TNF-a, IL-1b, NO, ROS production, prostaglandin E2 (PGE2), and phagocytosis. | Park et al. (2011) |
| Facilitate the downregulation of TLR4, NF-kB, p38 MAPK, c-Jun N-terminal kinase (JNK), and protein kinase B (AKT) phosphorylation. | ||||
| In vivo rat model of neuropathic pain | Inhibited microglial activation, reduced cytokine production and alleviate pain | Chang et al. (2022) | ||
| Chronic constriction injury (CCI)-induced injury | ||||
| Myricetin |
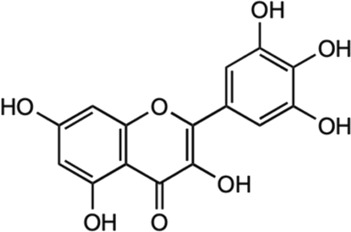
|
In vitro assay using mouse macrophage RAW 264.7 | Inhibited NO and pro-inflammatory cytokines. | Kim et al. (2013) |
| *LPS-stimulated cells | ||||
| Reduce expression of cyclooxygenase-2 (COX-2) and iNOS. | ||||
| (−)− Epicatechin |
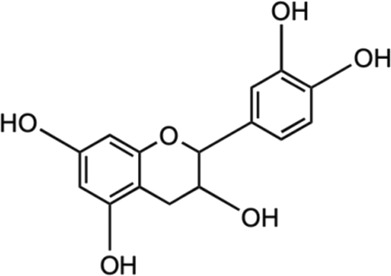
|
In vivo male C57BL/6 mice (wild type) and Nrf2 gene knockout (KO) mice | Reduced neutrophil infiltration and oxidative stress. | Cheng et al. (2016) |
| Facilitate activation of Nrf2 pathway, averted expression of heme oxygenase-1 protein, and reduced iron deposition. | ||||
| *Traumatic brain injury (TBI)-induced | ||||
| Quercetin |
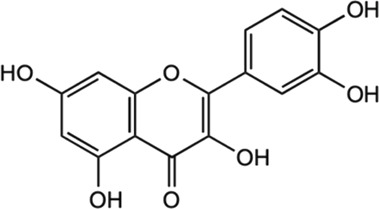
|
In vitro assay using SK-N-MC human neuroblastoma cell line In vivo male Sprague-Dawley (SD) rat |
Alleviated oxidative stress and inhibited cell apoptosis. | Bahar et al. (2017) |
| Exhibited antioxidant activity by apoptosis regulation, iNOS/NF-κB, and HO-1/Nrf2 related pathways. | ||||
| *Manganese (Mn)-induced neurotoxicity | ||||
| Isoquercitrin |
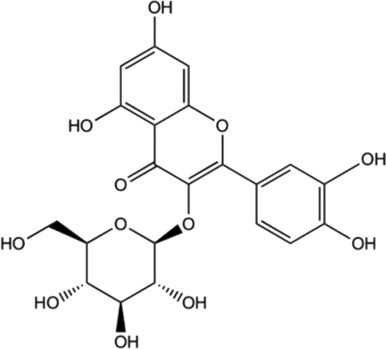
|
In vitro assay using mesenchymal stem cells | Exhibited ROS scavenging activities. | Li et al. (2016) |
| *ROS-Induced Damage | ||||
| In vivo male C57BL/6J mice | Attenuated impaired behaviors and loss of dopamine neurons, elevated expression of dopamine transporter and tyrosine hydroxylase. | Liu et al. (2021) | ||
| *1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced acute mouse model of PD | ||||
| Reduced the expression of pro-apoptotic signaling molecule Bax and supressing MPTP-triggered oxidative stress. | ||||
| In vivo male C57BL mice | Suppressed inflammatory responses and inhibited TLR4 and C5aR1 expression, as well as down-regulated MAPK signaling pathways. | Kim et al. (2022b) | ||
| *LPS-induced neuroinflammation | ||||
| Inhibited ROS generation. | ||||
| In vitro assay using mesenchymal stem cells | Exhibited ROS scavenging activities. | Li et al. (2016) | ||
| *ROS-Induced Damage | ||||
| Astragalin |
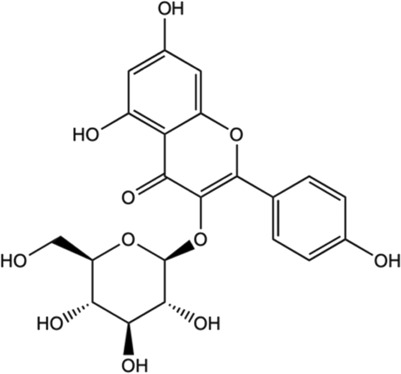
|
In vivo female C57BL/6 mice | Modulated inflammatory cascade; decreased release of INF-γ and IL-17 (pro-inflammatory cytokines) and increased for IL-10 (anti-inflammatory cytokine). | Liu et al. (2021) |
| *Neuropathy model | ||||
| Inhibited inflammatory pathway and impeded voltage-gated ion channels. | ||||
| Isothiocyanates (ITCs) | ||||
| Moringin |
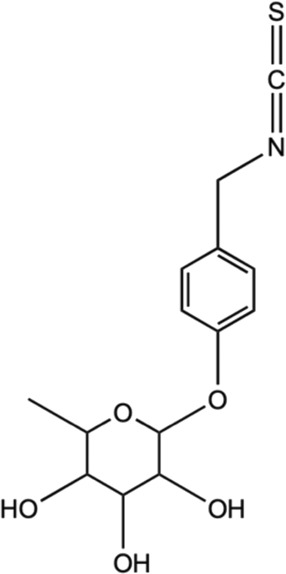
|
In vivo female C57BL/6 mice | Modulated inflammatory cascade; reduced production of INF-γ and IL-17 (pro-inflammatory cytokines) and increased IL-10 (anti-inflammatory cytokine). | Giacoppo et al. (2017) |
| Inhibited inflammatory pathway and impeded voltage-gated ion channels. | ||||
| *Neuropathy model | ||||
| Allyl isothiocyanates (AITC) |
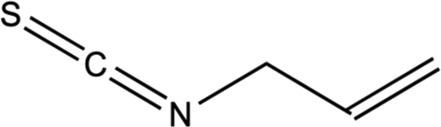
|
In vitro assay using C6 glioma, BV2 murine microglia, and N2a mouse neuroblastoma cells | Impeded with TNF-α formation in LPS-activated microglia and activation of JNK, NF-κB. | Subedi et al. (2017) |
| Alleviated inducing anti-apoptotic proteins and pro-apoptotic proteins to avert neuronal death. | ||||
