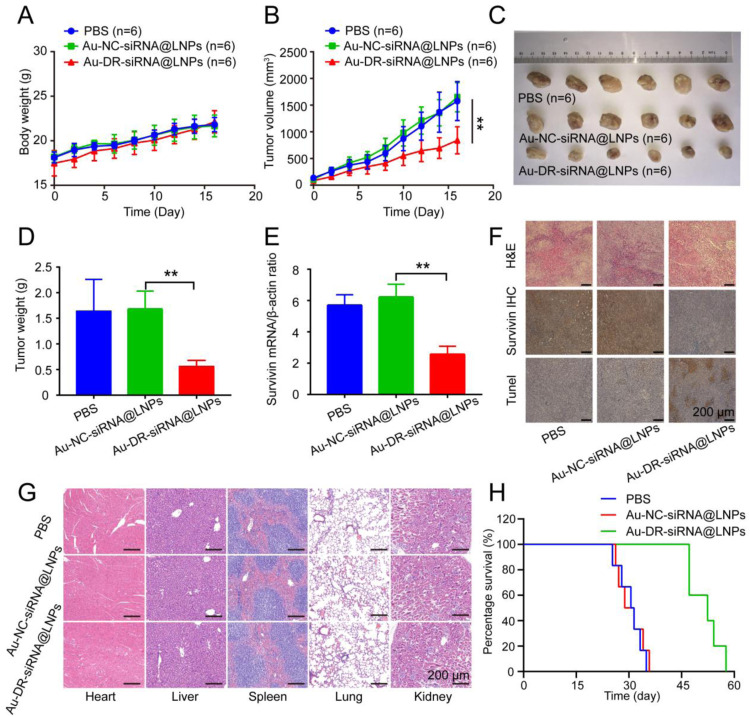
Fig. 6.
Antitumor effect of 4T1 tumour-bearing mice after tail vein administration of nanoparticles. (A) Body weights of 4T1 tumour-bearing mice treated with PBS, Au-NC-siRNA@LNPs, and Au-DR-siRNA@LNPs for 17 d (B) tumour volume of 4T1 tumour-bearing mice treated with PBS, Au-NC-siRNA@LNPs, and Au-DR-siRNA@LNPs for 17 d (C) Representative images of tumors after 17-d treatment with PBS, Au-NC-siRNA@LNPs, and Au-DR-siRNA@LNPs. (D) Average weights of tumors after 17-d treatments. (E) RT-PCR quantification of survivin gene expression in tumour tissues after 17-d treatments. (F) H&E, survivin immunohistochemical, and Tunel staining of tumour tissue sections in 4T1 tumour-bearing mice after 17-d treatments. Scale bar: 200 µm. (G) H&E histopathological analysis of major organ tissues in 4T1 tumour-bearing mice after 17-d treatments. Scale bar: 200 µm. (H) Survival curves of 4T1 tumour-bearing mice treated with PBS, Au-NC-siRNA@LNPs, and Au-DR-siRNA@LNPs. The data are presented as mean ± SD (n = 6). **P < 0.01 (One-way ANOVA analysis with Tukey's post-hoc test).