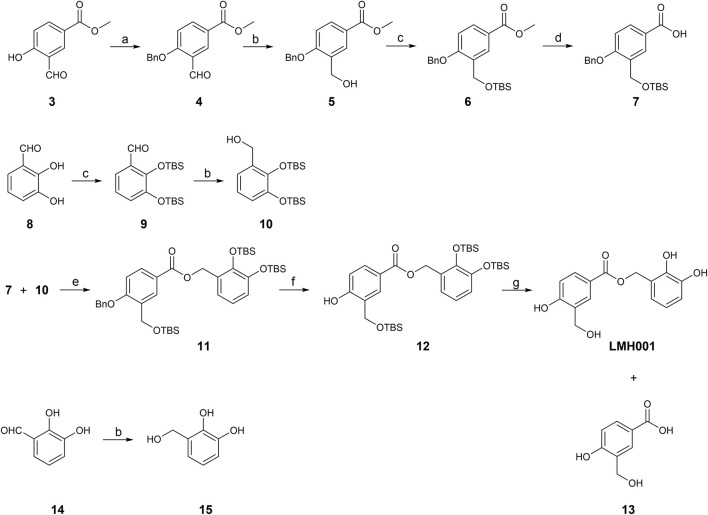
FIGURE 2.
Synthetic scheme of LMH001 and its two breakdown products (13 and 15). Reagents and conditions: (a) benzyl bromide, Cs2CO3, anhydrous DMF, rt, 7.5 h, quantitative; (b) NaBH4, MeOH, 0°C or 0°C to rt, 12 h, 5% for 15 and quantitative for 5 and 10 (c) TBSCl, imidazole, DCM, rt, 12 h, 64%–73% (for 6, 9); (d) DCM, 3 M NaOH in MeOH, rt, 18 h, 66%; (e) DCC, DMAP, DCM, rt, 24 h, 60%; (f) H2 (1 atm), 10% Pd/C, MeOH, rt, 1 h, 40%; (g) 1 M TBAF in THF, THF, 0°C to rt, 1 h, 6%.