Fig. 2.
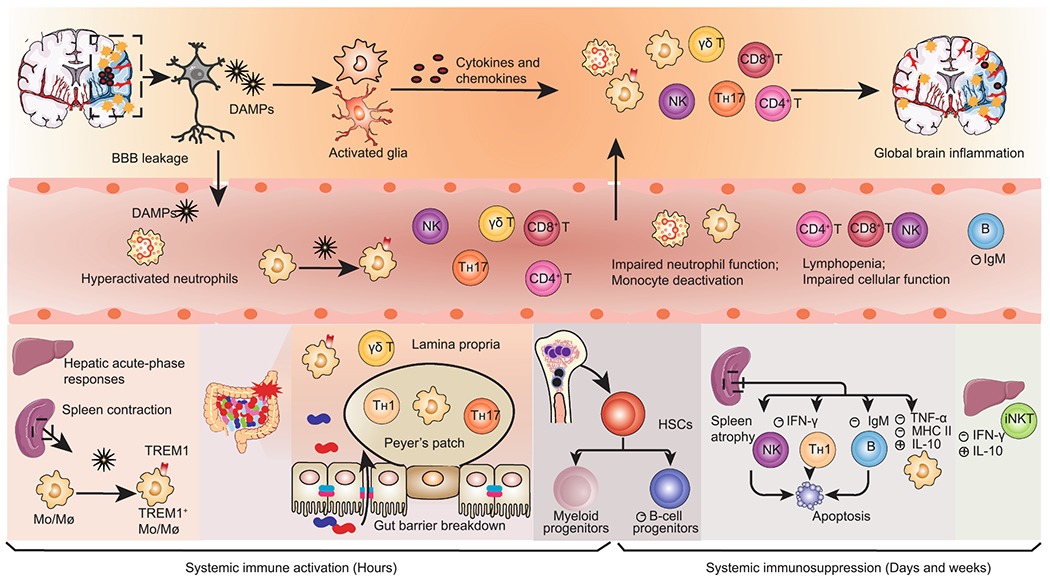
Dynamic alterations in systemic immunity after AIS. Ischemic neural cells release damage-associated molecular patterns (DAMPs) that trigger local inflammatory cascades, including glial activation and infiltration of peripheral immune cells. Simultaneously, DAMPs reach the circulation and elicit systemic immune activation, remarked by activation of peripheral innate immune cells and massive secretion of proinflammatory mediators in the liver, spleen, gut, and circulation. Splenic Mo/Mø (monocytes/macrophages) are rapidly deployed into the blood. Peripheral Mo/Mø display elevated TREM1+ expression in response to both brain-derived DAMPs and gut-derived bacterial components arising from gut leakage. Systemic immune activation is rapidly superseded by a long-lasting immunosuppression that encompasses spleen atrophy, lymphopenia and impaired function of both the innate and adaptive immunity. The loss of spleen marginal zone B cells causes a declined secretion of immunoglobulin M (IgM). The bone marrow displays enhanced myelopoiesis and deficient B lymphopoiesis, with the former contributing to early circulating neutrophilia and monocytosis and the latter favoring later immunosuppression. AIS, acute ischemic stroke; BBB, blood-brain barrier; CRP, C-reactive protein; TREM1, triggering receptor expressed on myeloid cells 1; HSCs, hematopoietic stem cells; iNKT, invariant natural killer T.
