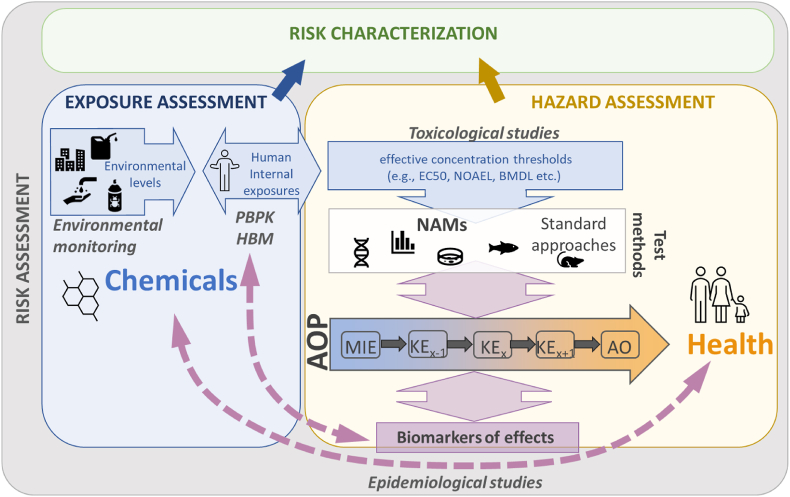
Fig. 1.
The place of the AOP framework in bridging the different components of next generation risk assessment, improving causal inference in exposure-health relationships in epidemiological studies, and identifying and validating biomarkers of effects. Abbreviations: HBM, human biomonitoring; PBPK, physiologically based pharmacokinetic modelling; NAMs, new approach methodologies; AOP, adverse outcome pathway; MIE, molecular initiating event; KE, key event; AO, adverse outcome; EC50, half maximal effective concentration; NOAEL, no observed adverse effect level; BMDL, benchmark dose level.