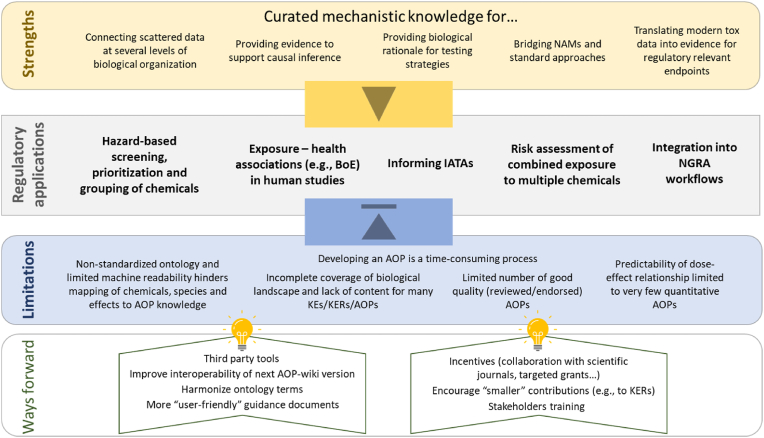
Fig. 2.
Strengths and limitations of AOPs as a tool to translate scientific data into regulatory relevant knowledge to support risk assessment . Five regulatory applications (light grey box) benefit from the curated and chemical-agnostic AOP-knowledge (light yellow box and yellow arrowhead), but the full adoption of AOPs is currently hindered by several limitations (light blue box and blue arrowhead). Some ongoing or proposed initiatives should help overcome the limitations in future (ways forward). Benefits, applications, limitations and ways forward are all commented in greater details in the manuscript. Abbreviations: AOP, adverse outcome pathway; IATAs, integrated approaches to testing and assessment; BoE, biomarkers of effect; KE, key events; KERs, key event relationships; NGRA, next generation risk assessment. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)