Fig. 3.
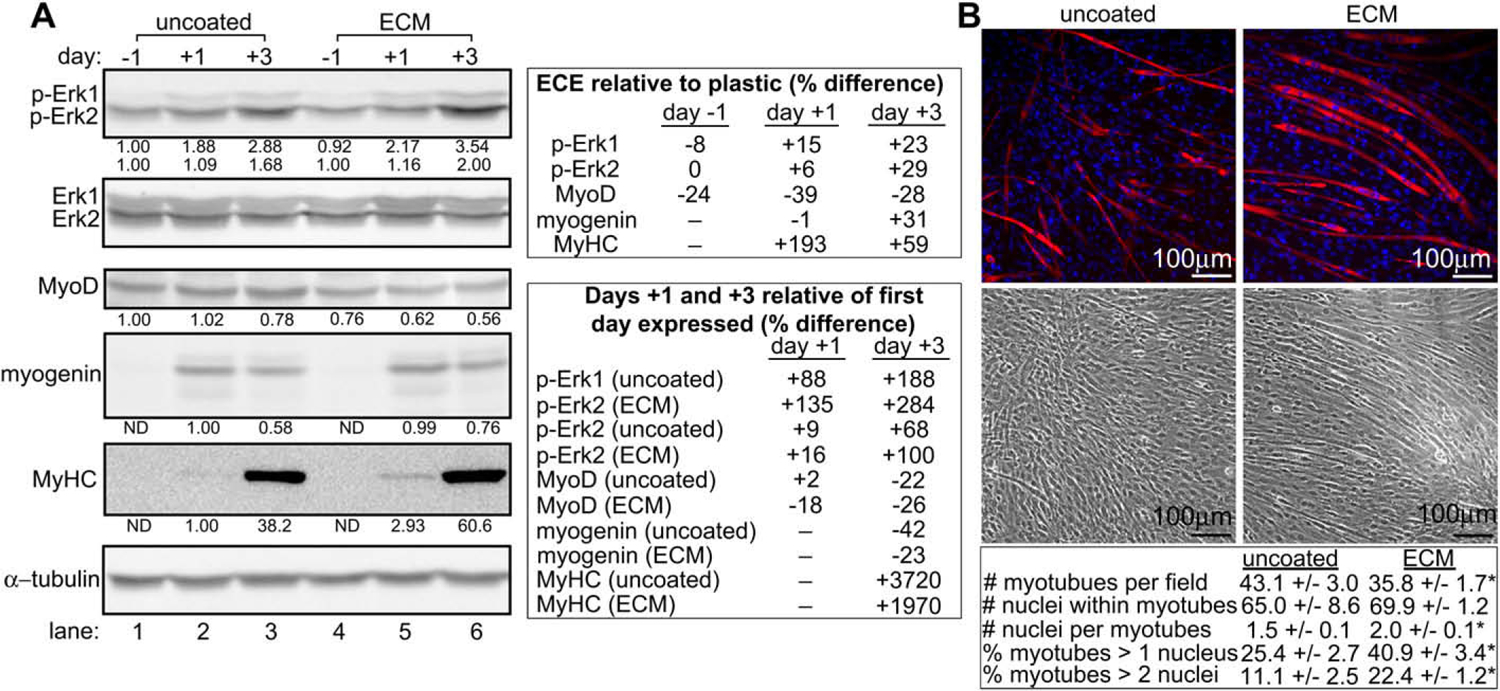
Cells grown on muscle ECM undergo enhanced Erk1/2 phosphorylation and myogenic differentiation over the course of 7 days. (A) Lysates from C2C12 cells grown on uncoated or skeletal muscle ECM extract-coated dishes were assayed for expression of phosphorylated Erk1/2 (p-Erk1/2) and markers of myogenic differentiation by Western analysis. Day numbers indicate the day of culture relative to the addition of myogenic differentiation medium on day 0. For densitometry in p-Erk1/2 blots, Erk1/2 values were normalized to α-tubulin. p-Erk1/2 values were then normalized to the adjusted Erk1/2 values, and the p-Erk1/2 values obtained from cells grown on uncoated dishes on day −1 were set to 1.00. For densitometry in remaining blots, values were normalized to α-tubulin. Values obtained from cells grown on uncoated dishes on the first day of their expression were set to 1.00. All bands are of the expected molecular weight. A representative experiment is shown. (B) C2C12 cells grown on uncoated or ECM-coated surfaces were assayed for the formation of myotubes by immunofluorescence microscopy. The number of myotubes per field and nuclei per myotube were compared between cells grown on uncoated and ECM-coated surfaces. * indicates a statistically different (p < 0.05) result compared to uncoated wells. Data are presented as average ± SEM.
