Fig 2.
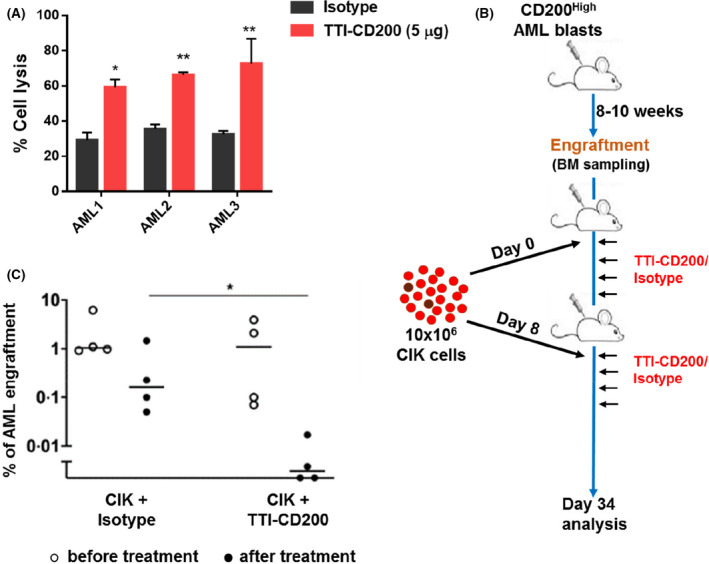
TTI‐CD200 sensitises AML cells towards CIK cell‐mediated lysis ex vivo and in vivo. (A) Bar graphs represent percentage of cell lysis of primary AML blast cells. Cells were pre‐treated with either TTI‐CD200 or isotype control (5 μ g/mL) for 1h, followed by co‐culture with CIK cells for 5h with E:T of 10:1. Other E:T ratios are shown in supplemental Fig. S5. Percentage of cell lysis was calculated using flow cytometry and shows the relative number of AML blasts recovered, following CIK cell co‐culture in TT‐CD200 and isotype‐treated groups. Data are mean ± 1SD from three experiments with * P ≤ 0·05 and ** P ≤ 0·01, analysed by Dunnett’s multiple comparison test. (B) Schematic plan for in vivo experiments. NSG mice were injected with primary CD200High AML blasts. Mice were monitored for 8‐10 weeks for AML engraftment by bone marrow sampling and subsequently injected with CIK cells every seventh day, followed by treatment with TTI‐CD200 or isotype (10mg/kg) on the day, and every two days for eight times. (C) Data represent percentage of AML engraftment in NSG mice as analysed through flow cytometry as the expression of hCD45+CD33+CD19‐. Every point represents an individual mouse. Horisontal bar represents the mean with *P ≤ 0·05, analysed by the Mann‐Whitney test.
