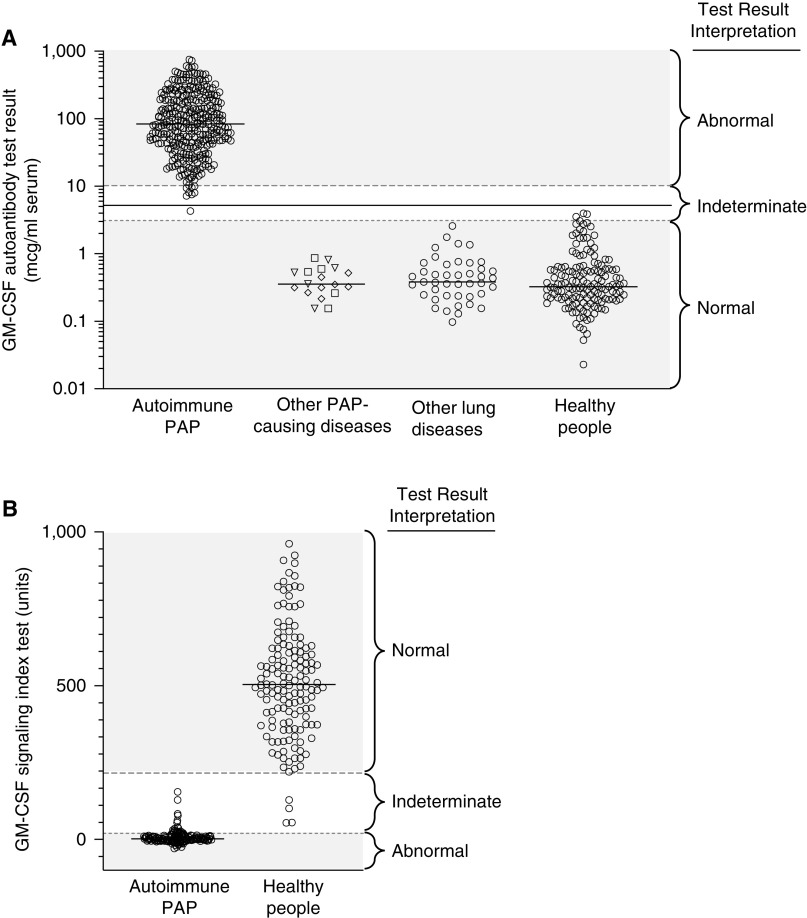
Figure 4.
Blood-based tests used to diagnose autoimmune pulmonary alveolar proteinosis (PAP). (A) Measurement of serum GM-CSF (granulocyte/macrophage colony–stimulating factor) autoantibody concentration by the serum GM-CSF autoantibody test. Shown are results for individuals with the indicated diseases and interpretive ranges for the test results. Each symbol represents the test result for one individual determined by ELISA using a patient-derived, affinity-purified, polyclonal GM-CSF autoantibody reference standard as previously reported (155). The reference ranges used for test result interpretation (normal, ⩽3.1 μg/ml; indeterminate, >3.1 to <10.2 μg/ml; and abnormal, ⩾10.2 μg/ml) were determined according to guidelines established by the Clinical and Laboratory Standards Institute (234), and updated on April 5, 2019, on the basis of results for 153 healthy individuals (median, 0.33 μg/ml; 90% confidence interval [CI], 0.3–0.4 μg/ml) and 339 patients with autoimmune PAP (median, 84 μg/ml; 90% CI, 10.2–499 μg/ml). Test results within the indeterminate range are typically confirmed by evaluation of GM-CSF signaling. (B) Measurement of GM-CSF signaling by the GM-CSF signaling index (GM-CSF-SI) test. Shown are results for individuals with autoimmune PAP and healthy people and the interpretive ranges for the test results. Each symbol represents the test result for one individual determined by incubating heparinized whole blood with recombinant human GM-CSF (10 ng/ml, 30 min) followed by flow cytometry to quantify phosphorylated STAT5. Results are expressed as GM-CSF-SI, calculated as the mean fluorescence intensity (MFI) in GM-CSF–stimulated cells minus the MFI of unstimulated cells divided by the MFI of unstimulated cells and multiplied by 100, as previously reported (75). The reference ranges used for test result interpretation (normal, ⩾216 units; indeterminate, >20 to <216 units; abnormal, ⩽20 units) were determined according to guidelines established by the Clinical and Laboratory Standards Institute (234) and updated on April 8, 2019, on the basis of results for 77 healthy individuals (median, 506 units; 90% CI, 483–564 units) and 80 patients with autoimmune PAP (median, 2.2 units; 90% CI, 0–4.2 units). STAT5 = signal transducer and activator of transcription 5.