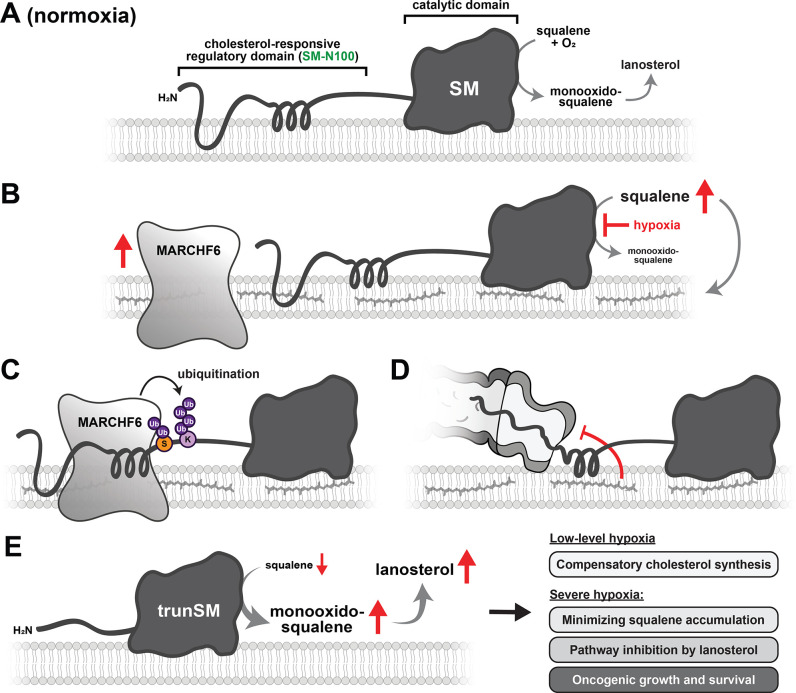
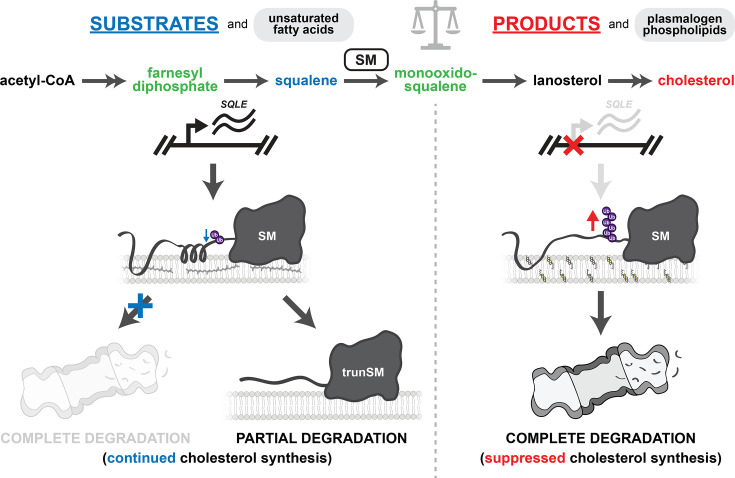
Figure 6. Model of hypoxia-induced SM truncation.
(A, B) Hypoxic conditions stabilize the E3 ubiquitin ligase MARCHF6 and inhibit SM-catalyzed conversion of squalene to monooxidosqualene, leading to squalene accumulation. (C) Increased MARCHF6 activity promotes ubiquitination of SM and its targeting to the proteasome. (D) Squalene impedes the complete proteasomal degradation of SM via a mechanism involving the SM-N100 domain, (E) yielding the constitutively active trunSM. During transient or low-level hypoxia, trunSM activity may facilitate continued cholesterol synthesis to compensate for the oxygen shortfall. During long-term or severe hypoxia, trunSM activity may reduce squalene-induced toxicity and promote downstream synthesis of lanosterol, which suppresses an early step of the cholesterol synthesis pathway. During pathophysiological hypoxia, cholesterol synthesis enabled by trunSM may contribute to oncogenic cell growth and survival.