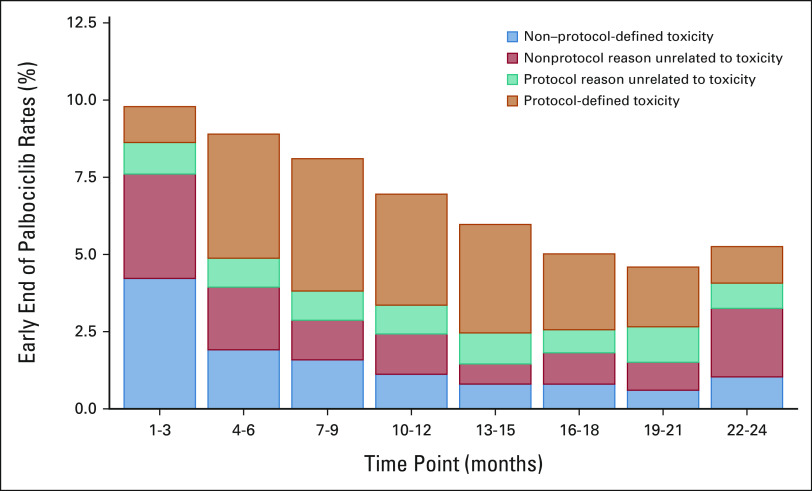
FIG 2.
Reasons for early palbociclib discontinuation, categorized by central study management teams as protocol-defined or non–protocol-defined, for palbociclib + ET patients by 3-month time points as presented to the IDMC in May 2020. The percentages in the 3-month periods refer to the number of patients who were still receiving palbociclib treatment up to the respective period. aThe increased rate of early discontinuation for nonprotocol reasons unrelated to toxicity in months 22-24 reflects administrative reasons early in the course of the study. ET, endocrine therapy; IMDC, Independent Data Monitoring Committee.