FIG 2.
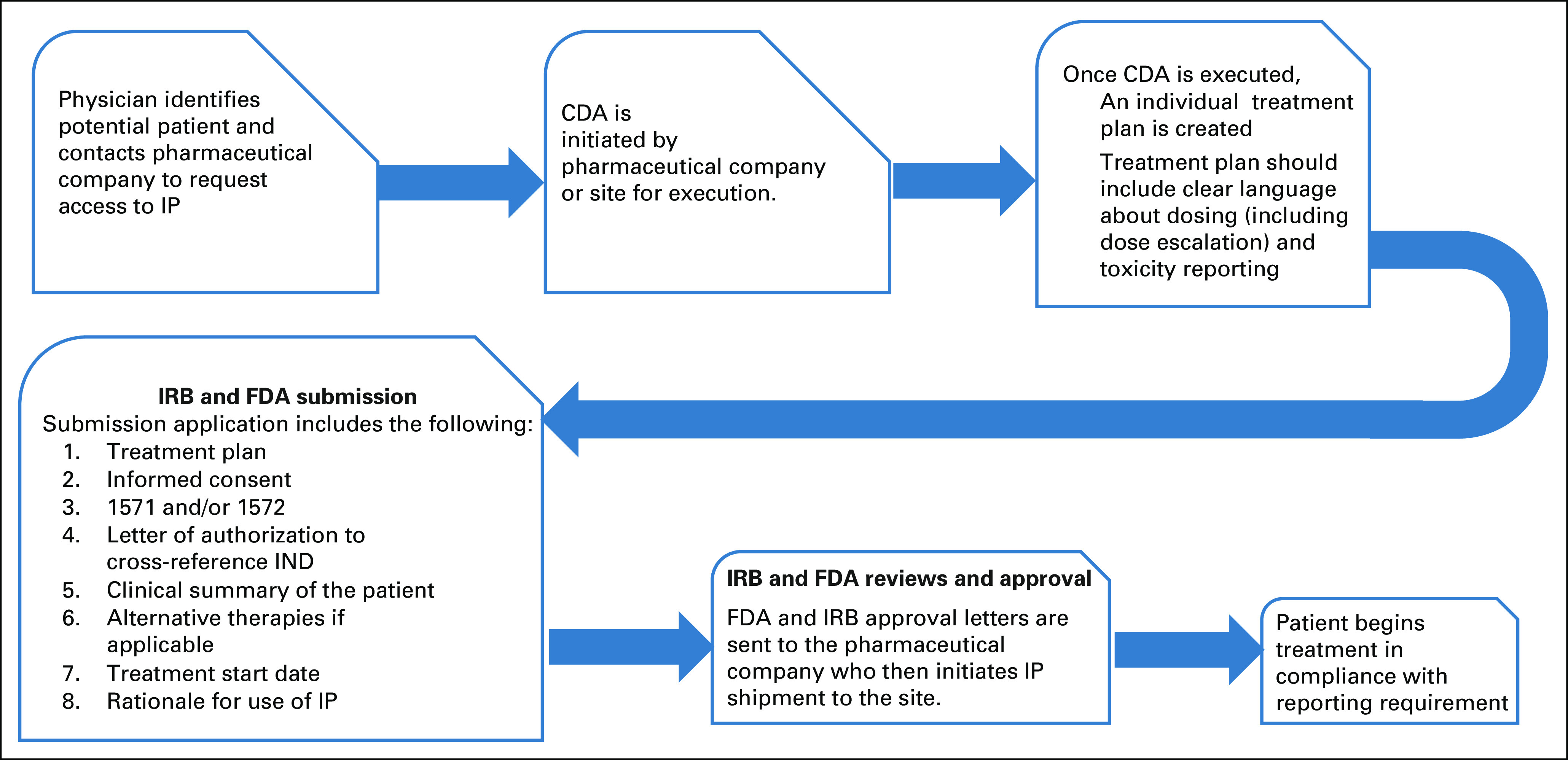
Schematic outlining the SPU approval process (single patient use IND regulatory process). CDA, Confidentiality Disclosure Agreement; FDA, US Food and Drug Administration; IND, investigational new drug; IP, investigational product; IRB, Institutional Review Board; SPU, single patient use.
