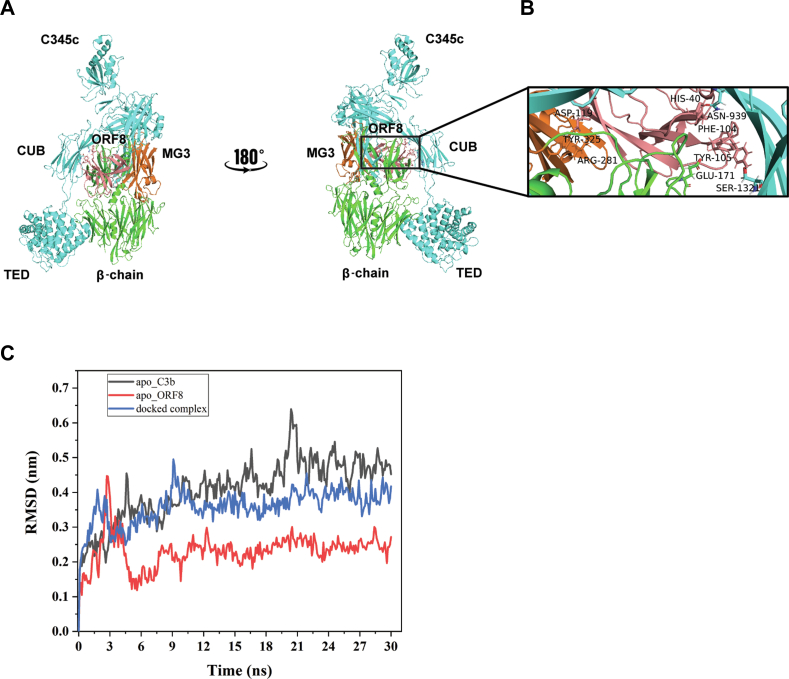
Figure 4.
In-silico model of C3b-ORF8 cocomplex.A, mini FH and FI were replaced from the structure C3b-mini FH-FI structure (PDB entry 5O32) and docked with ORF8 (PDB entry 7JTL). ORF8 (salmon) appears to partially occupy complement control protein (CCP) binding domain 2-3 on C3b. ORF8 is shown to make multiple contacts with the MG3 (orange) and MG2 domains of β-chain. B, zoomed view of interactions between the MG3 domain residues (orange), ARG-281 and TYR-325, with ASP-119 (ORF8). Also, interactions between His-40 (ORF8)/ASP-939 (α-chain C3b) and PHE-104 (ORF8)/GLU-171 (β-chain C3b), respectively, show proximity to the second cleavage site RS (1320-1321) of CUB domain in α-chain (cyan) of C3b. More information in Table S1. C, molecular dynamics simulation analysis confirms the stabilization of the docked complex (C3b-ORF8) as compared to the apo-C3b (uncomplexed). Root Mean Square Deviation (RMSD) values (nm) generated for 30 ns at 150 mM KCl solvent system are plotted for comparison using Gromacs simulation package (details provided in supporting information). FI, Factor I; FH, Factor H.