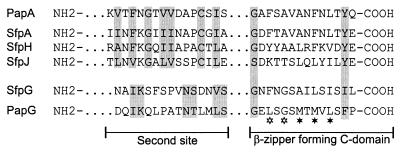
FIG. 2.
Conserved structural motifs within Sfp subunits. Putative amino acid sequences of the C-terminal β-zipper-forming motif as well as the second site of interaction between pilins and the periplasmic chaperone are compared to the respective motifs of PapA and PapG (17). The most conserved residues are indicated by shaded boxes, and the asterisks mark the alternating hydrophobic amino acids in the C domain (open asterisks stand for residues conserved in all pilins with the exception of SfpJ).