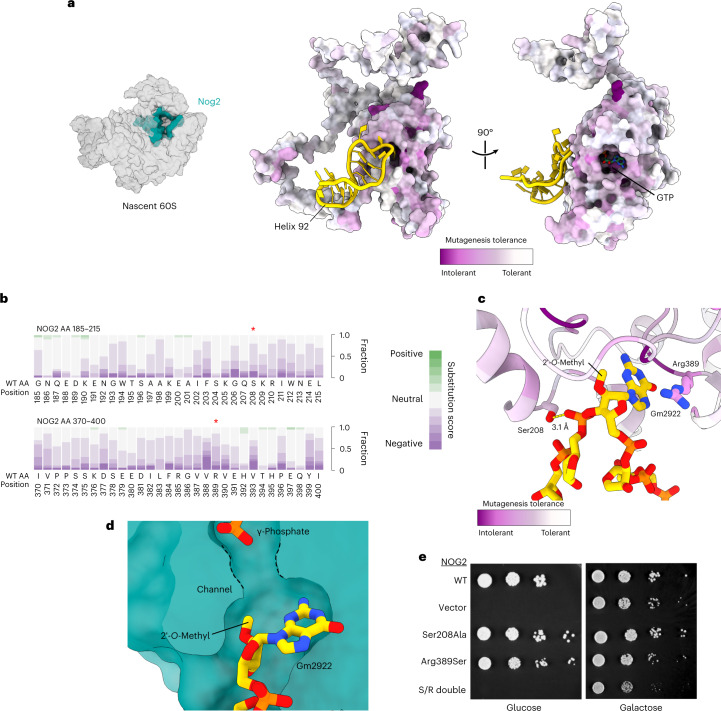
Fig. 1. Essential function of Nog2 depends on physical interaction with H92.
a, Left, structure of Nog2 relative to pre-60S (accession no. PDB 3JCT) in ‘crown view’. Right, Nog2 colored by per-residue average score of amino acid tolerance to mutation. H92 is colored gold. b, Cumulative per-residue fitness scores for all amino acid substitutions at H92-interacting regions of Nog2. Residues Ser208 and Arg389 are denoted by red asterisks. c, Cartoon representation of Nog2 residues interacting with H92, colored as in a. d, Cutaway surface representation of Nog2, with Gm2922 flipped into the active site channel. The γ-phosphate of GTP is also shown. e, Serial dilution assays to test complementation by the indicated NOG2 alleles in a repressible NOG2 strain (glucose, endogenous NOG2 repressed; galactose, expressed).