Fig. 4 |. Population-based and experimental validation of interactome-predicted drugs.
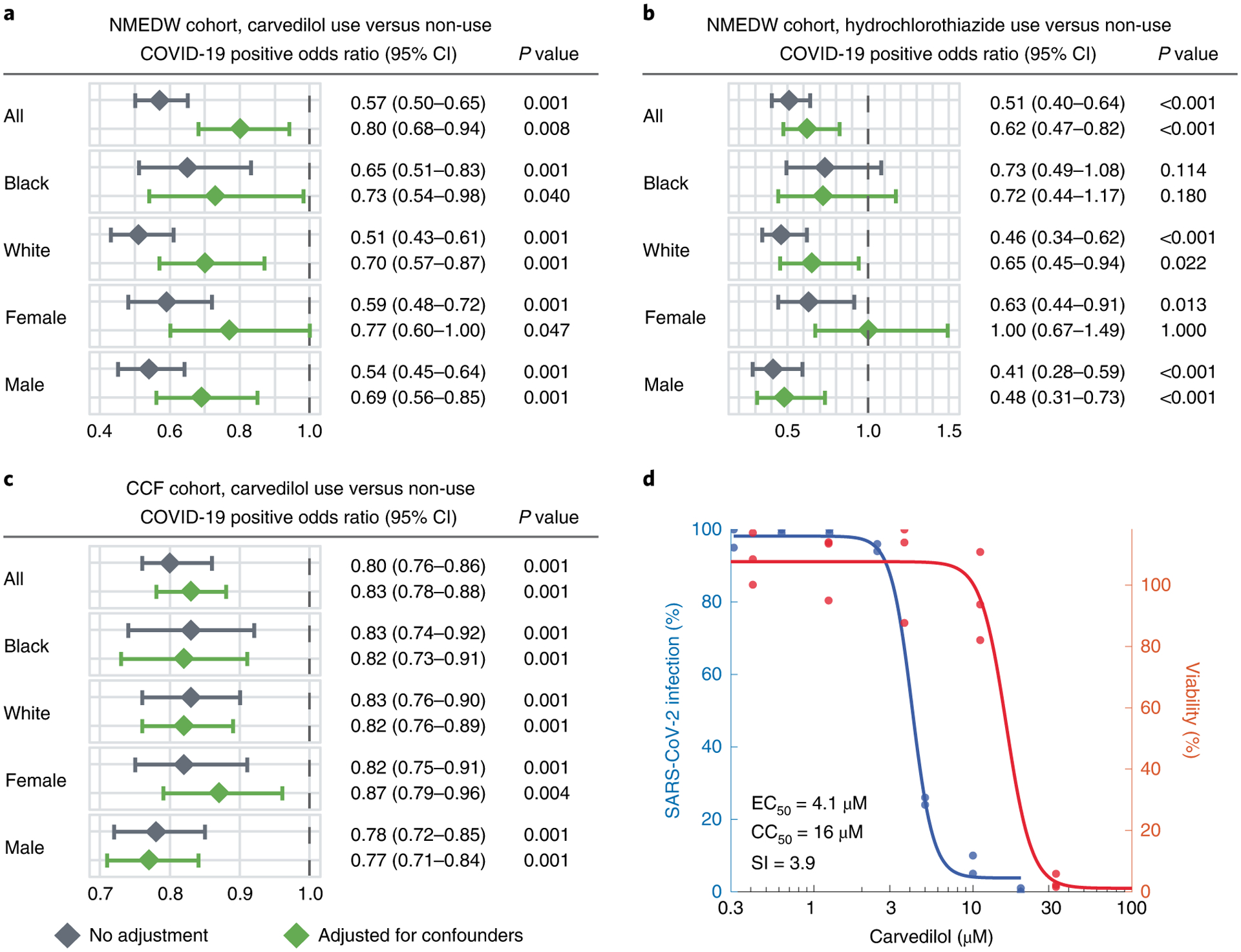
a–c, Drug–outcome evaluation using the NMEDW and CCF COVID-19 databases. Odds ratio was used to evaluate the carvedilol effect to the positive laboratory test result of COVID-19. Statistically significant at P < 0.05 and odds ratio < 1 indicate that the carvedilol user with lower odds of COVID-19 positive testing. The central diamond box denotes the odds ratio and the error bar denotes the 95% confidence interval. Patients were matched with propensity score using age, sex, race and other comorbidities (Table 1) to reduce various confounding factors. Statistics derived from cohort details shown in Table 1. d, Experimental validation of the anti-SARS-CoV-2 activity of carvedilol showed an EC50 value of 4.1 μM and low cell toxicity. CC50, half-maximal cytotoxic concentration; SI, selectivity index (SI = CC50/EC50).
