Figure 3. BLOC1S1 deficiency increases directly lysosome-lipid droplet interaction but not macroautophagy or chaperone-mediated lipophagy.
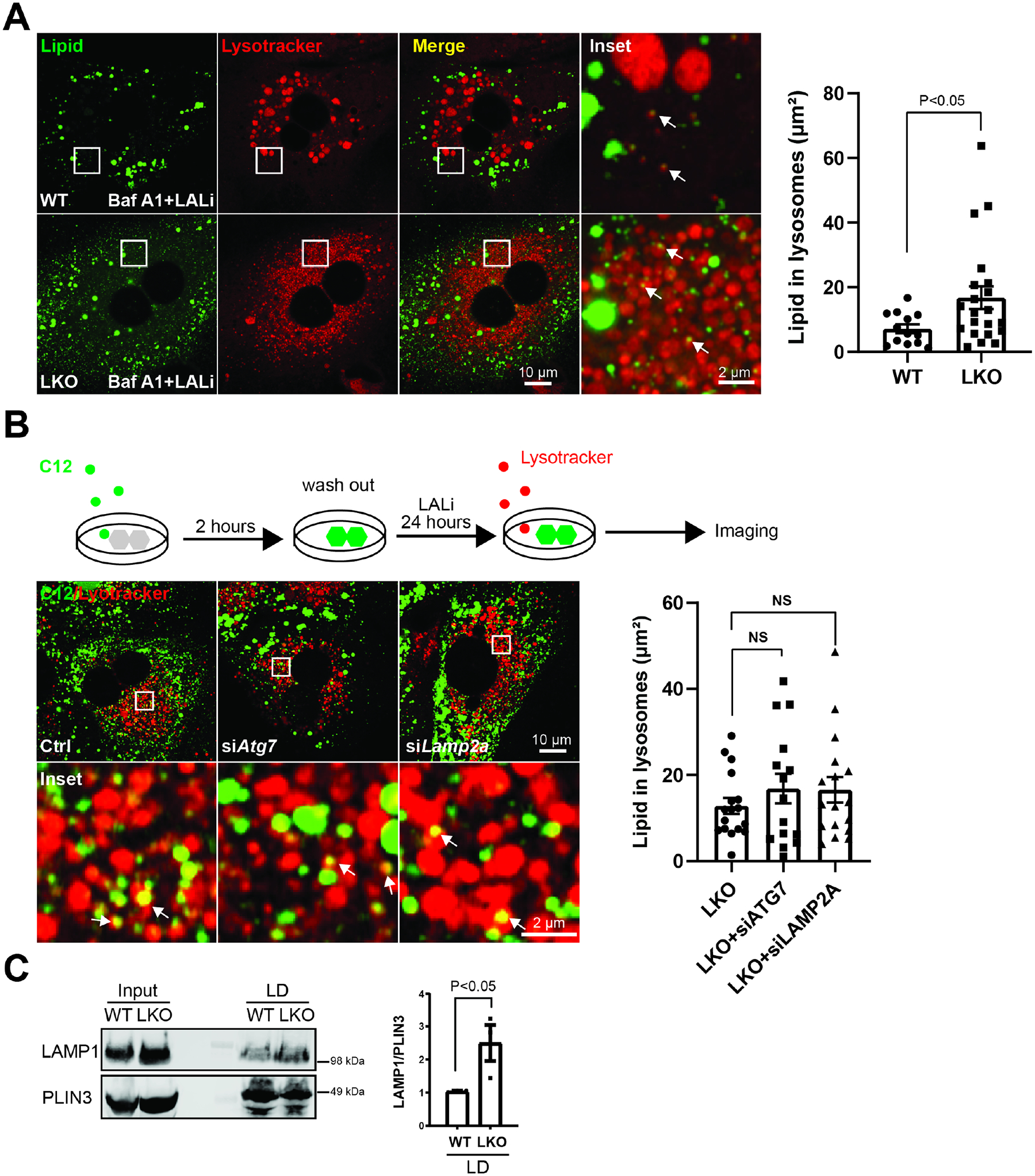
(A) Confocal microscopy to evaluate the effect of the disruption of autophagic flux by bafilomycin A1 to assess effect on LD accumulation in LALi treated primary hepatocytes in both groups. Lysosomes and lipid were stained with lysotracker (red) and BODIPY (green) respectively. The area of lipid inside lysosome were quantified in n > 15 cells from each group (B) Schematic diagram illustrates method to trace lysosome degradation of BODIPY labeled C12 long chain fatty acid in primary hepatocytes (upper panel). Confocal image following knockdown of macroautophagy (siATG7)/CMA machinery (siLAMP2A) to assess effect on lipid transfer to lysosomes in LKO hepatocytes (lower panel). Lysosomes were visualized by Lysotracker (red). The area of lipid inside lysosome was quantified in n > 15 cells from each group. (C) Lipid droplets were isolated from livers of WT and LKO mice after 24 hours fasting followed by immunoblot analysis of LAMP1 binding to lipid droplets. PLIN3 reflects the mass of LDs. Scale bars shown in image panels.
