Fig. 1.
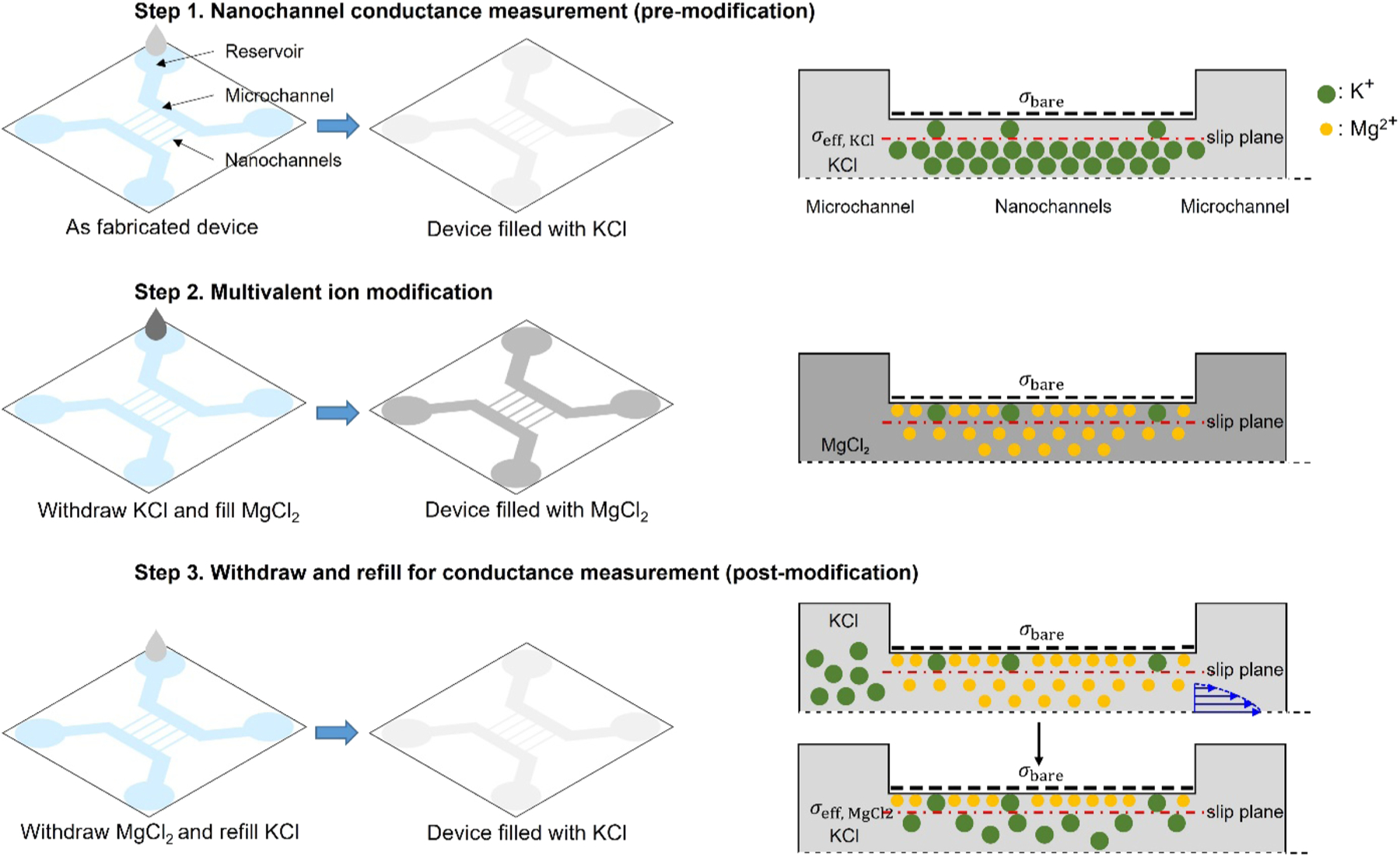
Surface modification process. The left panel describes how the electrolyte solution was introduced into the nanofluidic devices and the right panel presents schematics showing the changes of ion distribution inside the nanochannel (cross-section view along the channel). Step 1: Nanochannel conductance measurement before multivalent ion modification. A nanofluidic device was filled from one side of the microchannels with 10−6 M KCl. Once the microchannel was wet, the nanochannels were filled spontaneously by capillary force. From the measured conductance, σeff, KCl was calculated as a baseline. Step 2: Surface modification by multivalent ions. The KCl solution in the nanofluidic device was withdrawn by a vacuum pump and the device was filled with multivalent electrolyte (MgCl2 is shown as an example) having different concentrations. Step 3: Conductance measurement after modification. The mobile multivalent cations outside the slip plane were replaced with 10−6 M KCl by repeating withdrawal and refilling process multiple times. This step was monitored by measuring the nanochannel conductance every four withdraw-and-refill cycles. If the measured conductance was not saturated to a stable value (lower than the baseline), this step was repeated. σeff, MgCl2 was calculated from the saturated nanochannel conductance value after the modification.
