Optimization of reaction condition for 1,3 diones and aryl diazonium saltsa.
| ||||
|---|---|---|---|---|
| Entry | Catalyst (mol%) | Base (equivalent) | Solvent | Yieldb (%) |
| 1 | Eosin Y (5 mol%) | DIPEA (2) | ACN | 80 |
| 2 | Eosin Y (5 mol%) | DIPEA (2) | DMF | 65 |
| 3 | Eosin Y (5 mol%) | DIPEA (2) | Toluene | ND |
| 4 | Eosin Y (5 mol%) | DIPEA (2) | 1,4-Dioxane | 70 |
| 5 | Eosin Y (5 mol%) | DIPEA (2) | DCM | ND |
| 6 | Eosin Y (2 mol%) | DIPEA (2) | ACN | 80 |
| 7 | Eosin Y (2 mol%) | DIPEA (1) | ACN | 92 |
| 8 | — | DIPEA (1) | ACN | 20 |
| 9c | Eosin Y (2 mol%) | — | ACN | 25 |
| 10 | Eosin Y (2 mol%) | K3PO4 (1) | ACN | 58 |
| 11 | Ru(bpy)3Cl2 (2 mol%) | Et3N (1) | ACN | 71 |
| 12 | Eosin Y (2 mol%) | Et3N (1) | ACN | 25 |
| 13d | Eosin Y (2 mol%) (in dark, 8 h) | DIPEA (1) | ACN | 20 |
| 14 | Eosin Y (10 mol%) | DIPEA (1) | ACN | 84 |
| 15 | Methylene Blue (2 mol%) | DIPEA (1) | ACN | 67 |
| 16 | Rose Bengal (2 mol%) | DIPEA (1) | ACN | 74 |
| 17 | Methylene Blue (2 mol%) | DIPEA (1) | ACN | NR |
| 18 | Rose Bengal (2 mol%) | DIPEA (1) | H 2 O | 85 |
| 19 | Rose Bengal (2 mol%) | — | H2O | NR |
| 20 | Eosin Y | DIPEA (1) | H2O | 70 |
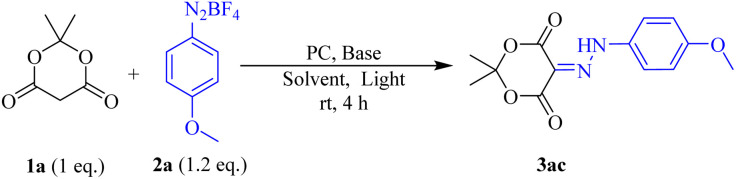
Reaction condition: 1,3-dione 1a (0.2 mmol), aryl diazonium salts (0.24 mmol), solvent (2.0 mL), room temperature, under 40 W Blue LEDs irradiation for 4 h.
Isolated yield.
Without base.
1,3-Dione 1a (0.2 mmol), aryl diazonium salts (0.24 mmol), solvent (2.0 mL), room temperature in dark condition.
