Abstract
Objectives
Georgia introduced remdesivir for the treatment of COVID-19 in December 2020. We evaluated the real-world effect of remdesivir on mortality and the need for mechanical ventilation among inpatients with COVID-19.
Methods
The study included 346 remdesivir recipients and 346 controls not receiving remdesivir selected through propensity score matching based on age, gender, presence of any chronic comorbid condition, and oxygen saturation at admission. Factors associated with in-hospital mortality and the need for mechanical ventilation were assessed in a multivariable logistic regression model.
Results
The groups were comparable by age, gender, comorbidities, and baseline oxygen saturation. Among 346 remdesivir recipients, 265 (76.6%) received a generic formulation of the drug. Eight (2.3%) patients died in the remdesivir group and 18 (5.2%) in the control group (P = 0.046). In the multivariable analysis, remdesivir was associated with non-statistically significant reduced odds of death (odds ratio: 0.39, 95% confidence interval: 0.14-1.04, P = 0.06). Significantly fewer patients in the remdesivir group required mechanical ventilation compared to controls: 2.9% vs 6.4% (P = 0.03). Statistically significant difference was maintained in multivariable analysis (odds ratio: 0.40, 95% confidence interval: 1.04-5.60, P = 0.04).
Conclusion
Borderline reduction in the odds of death and statistically significant decrease in the need for mechanical ventilation support use of remdesivir in hospitalized patients with COVID-19.
Keywords: COVID-19, Remdesivir, Mortality, Mechanical Ventilation, Georgia, Eastern Europe
Introduction
Remdesivir, a direct-acting nucleotide analog and RNA-dependent RNA-polymerase inhibitor, is the first antiviral drug authorized for the treatment of COVID-19 in the United States and European Union [1,2]. Authorizations followed the findings of adaptive COVID-19 treatment trial (ACTT-1), showing reduced time to recovery among remdesivir recipients [3]. ACTT-1 also showed a trend toward reducing mortality, but the difference did not reach statistical significance [3]. Neither did the World Health Organization (WHO) Solidarity trial demonstrate the survival benefit of remdesivir [4]. Real-world data on the impact of remdesivir on mortality yielded conflicting results so far, with several large observational studies indicating significant reduction in mortality, while others failed to show such benefit [5], [6], [7], [8], [9].
Georgia, an independent Eastern European country, was hit hard by the COVID-19 pandemic, with cumulative 415,000 infections and 4000 deaths reported per million population as of April 25, 2022 [10]. Georgia was one of the first resource-limited countries to recommend remdesivir for the treatment of hospitalized patients in December 2020. The country procured a generic formulation of the drug (Desrem, Mylan, India), but because of pandemic-related shipment delays, the brand product (Veklury, Gilead, USA) was also available for a short period of time through a donation from Gilead Sciences.
The primary objective of the study was to evaluate the real-world effect of remdesivir on mortality among hospitalized patients with COVID-19 in Georgia. The secondary objective was to assess the impact of remdesivir on the new need for mechanical ventilation.
Methods
National protocols on COVID-19 management
Management of patients with COVID-19 in Georgia is governed by national guidelines first approved by the Ministry of Health in April 2020 and last updated in December 2021 [11]. Hospitalization criteria have been changing depending on the epidemiological situation in the country and the burden on the healthcare system during outbreak waves. In general, hospitalization was strongly recommended for patients with severe COVID-19 disease (e.g., oxygen saturation on room air <94%, severe pneumonia with >50% infiltration of lung tissue). Patients with moderate COVID-19 disease (e.g., oxygen saturation on room air ≥94%, pneumonia with ≤50% infiltration of lung tissue) were also eligible for hospitalization, especially those at higher risk of disease progression, including people aged ≥65 years, presence of comorbid conditions such as cardiovascular disease, chronic lung disease, diabetes mellitus, cancer, obesity, chronic kidney disease, and immunocompromised conditions.
The December 2020 update of national guidelines recommended remdesivir for the treatment of hospitalized patients with COVID-19 with oxygen saturation <94% on room air and for patients at high risk of disease progression (age ≥65, chronic comorbid conditions). Treatment with remdesivir is recommended to be initiated within 10 days of symptom onset (or within 10 days of diagnosis if initially asymptomatic) with the following scheme: a bolus dose of 200 mg on day 1 and 100 mg daily on days 2 to 5. Treatment can be expanded to 10 days. According to the national COVID-19 treatment guideline, using remdesivir was not recommended in patients with hypersensitivity reactions to the medicine or any of its components, in patients with estimated glomerular filtration rate <30 ml/min per 1.73 m2, or patients with liver enzyme level elevations >5 times the upper limit of normal. Using remdesivir was not recommended during pregnancy unless the potential benefit outweighed the potential risk.
According to the national guidelines, the standard of care consists of symptomatic treatment, prophylactic anticoagulation for every hospitalized patient, and corticosteroids for patients requiring supplemental oxygen.
Study design and participants
We conducted a comparative effectiveness retrospective cohort study at the Infectious Diseases, AIDS and Clinical Immunology Research Center (IDACIRC) in Tbilisi, Georgia. This 100-bed hospital is the country's referral institution for infectious diseases, with 75% of its capacity devoted to caring for patients with COVID-19 during the pandemic.
The cohort included adults (age ≥18 years) with laboratory-confirmed COVID-19 (positive nasopharyngeal antigen or polymerase chain reaction test) first hospitalized at IDACIRC for at least 72 hours between November 01, 2020, and August 15, 2021, and not requiring mechanical ventilation at the time of hospitalization. The remdesivir arm of the study included consecutive patients who received at least one dose of the drug. Controls were selected through propensity score matching with 1:1 ratio based on age, gender, presence of any chronic comorbid condition, and oxygen saturation on room air at the time of hospitalization. Patients from both the remdesivir and control group were included during the same timeframe (November 2020-August 2021). The differences in the prescription of the drug were due to eligibility criteria, prioritization due to supply shortages, drug stock-out, and physicians’ discretion.
Statistical analysis
Primary outcome of interest was in-hospital mortality, and the secondary outcome of interest was the need for mechanical ventilation (combined non-invasive and invasive ventilation). Patients were followed from their admission until discharge or death during hospitalization. For assessing secondary outcomes, data were censored at the date of the start of mechanical ventilation. All data were extracted from the electronic medical records system that contained detailed information on every hospitalization. Bivariate comparisons were tested using Pearson's chi-square or Fisher's exact test as appropriate. Factors associated with in-hospital mortality and the need for mechanical ventilation were assessed in a multivariable logistic regression model. The multivariable regression model included all variables used for propensity score matching (baseline age, gender, pre-existing comorbidities, and oxygen saturation), including baseline laboratory values for neutrophils, lymphocytes, platelet count, C-reactive protein, d-dimer, and ferritin. To account for potential impact of circulating viral strain on outcomes of interest regression models were also adjusted for hospital admission periods, categorized by dominant SARS-CoV-2 strain circulating in Georgia: pre-variants of concern period (November 2020-January 2021), Alpha strain period (February-June 2021), and Delta strain period (July-August, 2021) [12] We performed sub-group analysis for patients who received generic remdesivir with propensity score-matched controls. All statistical analyses were performed using SAS 9.4. A P-value of <0.05 was considered statistically significant.
Results
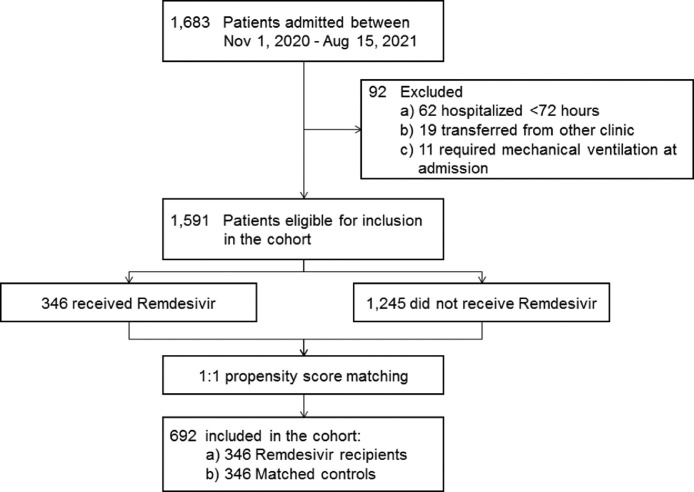
A total of 1683 adults with laboratory-confirmed COVID-19 were admitted to IDACIRC between November 01, 2020, and August 15, 2021. Among them, 1591 were eligible for inclusion in the cohort. A total of 346 patients received remdesivir, and all were included in the analysis, with 346 propensity score-matched controls selected among those not receiving remdesivir (Figure 1 ). The two groups were comparable in terms of age, gender, pre-existing comorbidities, and baseline oxygen saturation at the time of hospitalization (Table 1 ). No statistically significant differences were observed by individual comorbid conditions. The most common comorbid conditions in both groups were hypertension (32.4% in remdesivir group and 30.7% in control group, P = 0.62). There were significant differences in some of the baseline laboratory markers, including white blood cells, platelets, d-dimer, and ferritin (Table 1). More remdesivir patients had a platelet count of <150 × 109/l (26.6% vs 17.6%, P = 0.005), while more controls had d-dimer >1 mcg/ml (15.0% vs 9.8%, P = 0.04). Patients were hospitalized after median of 5 (interquartile range [IQR]: 3-7) days since symptom onset, including 5 (IQR: 3-6) days for remdesivir group and 6 (IQR: 4-9) days for control group (P <0.0001). Differences were also found in admission time periods. In both groups, most of the patients were admitted during the dominance of the Alpha strain, and 30.2% of the total cohort was hospitalized during the dominance of the Delta strain (Table 1). No statistically significant differences were found in terms of vaccination status. Overall, 18 (2.6%) participants had any history of vaccination at the time of admission; among them, only 4 (0.6%) were fully vaccinated (Table 1).
Figure 1.
Study flow diagram.
Table 1.
Baseline population characteristics at the time of hospitalization and COVID-19-related use of therapeutics (n = 652).
| Remdesivir (n = 346) | No remdesivir (n = 346) | P-value | |
|---|---|---|---|
| Age in years, median (IQR) | 60 (50-69) | 60 (52-69) | 0.61 |
| Age ≥65, n (%) | 115 (33.2) | 121 (35.0) | 0.63 |
| Women, n (%) | 150 (43.3) | 154 (44.5) | 0.76 |
| Any pre-existing comorbidity, n (%)a | 228 (65.9) | 230 (66.5) | 0.87 |
| Hypertension | 112 (32.4) | 106 (30.7) | 0.62 |
| Heart diseases | 45 (13.0) | 37 (10.7) | 0.35 |
| Diabetes mellitus | 45 (13.0) | 51 (14.7) | 0.58 |
| Chronic infectious diseases | 14 (4.0) | 13 (3.8) | 0.84 |
| Obesity | 12 (3.5) | 5 (1.4) | 0.09 |
| Cancer | 10 (2.9) | 6 (1.7) | 0.31 |
| Chronic obstructive pulmonary disease | 8 (2.3) | 9 (2.6) | 0.81 |
| Other | 39 (11.3) | 30 (8.7) | 0.25 |
| SpO2, median (IQR) | 95 (93-96) | 95 (93-96) | 0.55 |
| SpO2, <94%, n (%) | 110 (31.8) | 108 (31.2) | 0.87 |
| Symptom duration, median days (IQR) | 5 (3-6) | 6 (4-9) | <0.0001 |
| White blood cell count, median X 109/l (IQR) | 5.1 (4.0-6.1) | 5.3 (4.3-6.7) | 0.02 |
| Neutrophil count, median X 109/l (IQR) | 3.2 (2.4-4.1) | 3.3 (2.5-4.4) | 0.09 |
| Lymphocyte count, median X 109/l (IQR) | 1.1 (0.8-1.5) | 1.2 (0.9-1.6) | 0.05 |
| Neutrophil-to-lymphocyte ratio, median (IQR) | 2.7 (1.8-3.9) | 2.6 (1.7-4.0) | 0.65 |
| Neutrophil-to-lymphocyte ratio >3.3, n (%) | 121 (35.0) | 119 (34.4) | 0.87 |
| Platelet count, median X 109/l (IQR) | 180 (146-209) | 200 (162-220) | <0.0001 |
| Platelet count <150 × 109/l, n (%) | 92 (26.6) | 61 (17.6) | 0.005 |
| C-reactive protein, median mg/l (IQR) | 24.5 (12.0-45.0) | 27.1 (13.4-48.1) | 0.30 |
| C-reactive protein ≥40 mg/l, n (%) | 104 (30.1) | 118 (34.1) | 0.25 |
| D-dimer, median mcg/ml (IQR) | 0.47 (0.34-0.67) | 0.52 (0.39-0.75) | 0.0007 |
| D-dimer >1.0 mcg/ml, n (%) | 34 (9.8) | 52 (16.0) | 0.04 |
| Ferritin, median mcg/l (IQR) | 182 (93-302) | 235 (114-340) | 0.03 |
| Ferritin >500 mcg/l, n (%) | 36 (10.4) | 45 (13.0) | 0.25 |
| Time period of admission by dominant strain, n (%) | |||
| Pre-Variants of Concern strains (November 2020-January 2021) | 34 (9.8) | 94 (27.2) | <0.0001 |
| Alpha strain (February-June 2021) | 187 (54.1) | 168 (48.5) | |
| Delta strain (July-August 2021) | 125 (36.1) | 84 (24.3) | |
| History of vaccination, n (%) | |||
| Fully vaccinated | 2 (0.6) | 2 (0.6) | 1.00 |
| Partially vaccinated | 9 (2.6) | 5 (1.4) | 0.28 |
| Therapeutics used during hospitalization, n (%) | |||
| Dexamethasone | 158 (45.7) | 149 (43.1) | 0.49 |
| Low-molecular-weight heparin | 322 (93.1) | 317 (91.6) | 0.47 |
| Antibiotics | 98 (28.3) | 110 (31.8) | 0.32 |
IQR, interquartile range; SpO2, oxygen saturation on room air.
Some patients had multiple comorbidities; among 228 remdesivir recipients with any comorbidity the total number of comorbidities was 285; among 230 patients with any comorbidity, the total number of comorbidities was 257.
No statistically significant differences were observed in the prescription of therapeutics other than remdesivir. Over the course of disease, 307 patients were prescribed dexamethasone, including 158 (45.7%) remdesivir recipients and 149 (43.1%) in the control group (P = 0.49). Low-molecular-weight heparin was prescribed to 93.1% of remdesivir recipients vs 91.6% among control group (P = 0.47). Use of antibiotics was documented in 28.3% of remdesivir recipients and 31.8% among controls (P = 0.17) (Table 1).
Among 346 remdesivir recipients, 265 (76.6%) received the generic formulation (Desrem, Mylan, India) and 81 (23.4%) the brand product (Velkury, Gilead, USA).
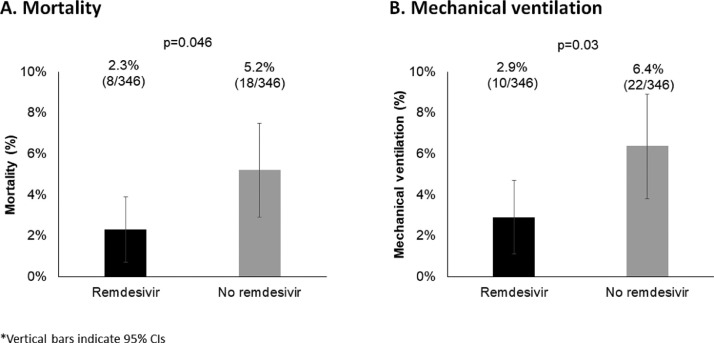
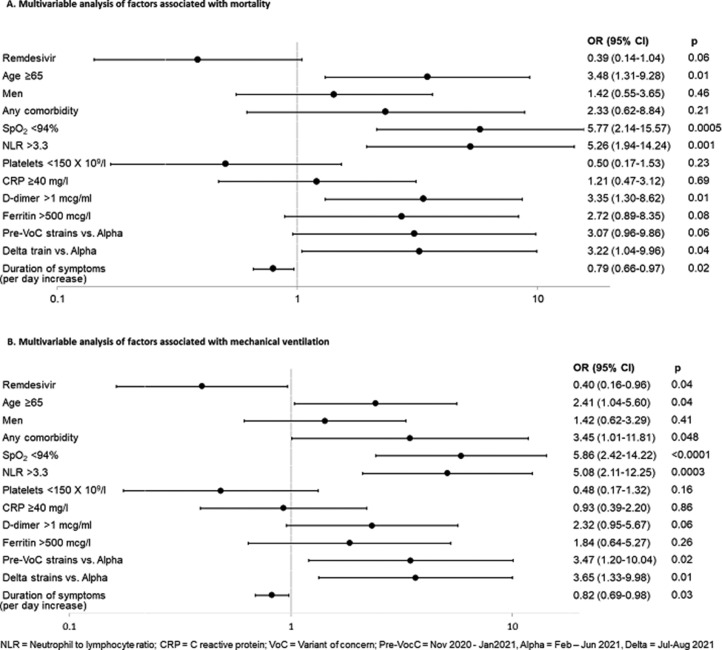
After the median 9 days of hospital stay (remdesivir: 9 [IQR: 7-12] days; control: 9 [IQR 6-12] days, P = 0.66), 8 (2.3%) patients died in the remdesivir group, compared to 18 (5.2%) in the control group (P = 0.046) (Figure 2 a). In the multivariable analysis, remdesivir was associated with reduced odds of death with borderline statistical significance: odds ratio (OR): 0.39, 95% confidence interval (CI): 0.14-1.04, P-value = 0.06 (Figure 3 a). Factors significantly associated with increased odds of mortality included: age ≥65 (OR: 3.48, 95% CI: 1.31-9.28, P = 0.01), baseline oxygen saturation <94% (OR: 5.77, 95% CI: 2.14-15.57, P = 0.0005), neutrophil-to-lymphocyte ratio >3.3 (OR: 5.26, 95% CI: 1.94-14.24, P = 0.001), d-dimer >1.0 mcg/ml (OR: 3.35, 95% CI: 1.30-8.62, P = 0.01), hospital admission during dominance of Delta strain vs Alpha (OR: 3.22, 95% CI: 1.04-9.96, P = 0.04). Duration of symptoms was associated with reduced odds of mortality (OR: 0.79, 95% CI: 0.66-0.97, P = 0.02).
Figure 2.
Primary and secondary outcome analyses (n = 692).
CI, confidence interval.
Figure 3.
Multivariable analysis of factors associated with primary and secondary outcomes (n = 692).
CI, confidence interval; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; SpO2, oxygen saturation on room air; VOC, variants of concern.
Overall, 32 patients required mechanical ventilation, including 11 requiring invasive ventilation (four in remdesivir and seven in control groups). Significantly fewer patients in the remdesivir group required mechanical ventilation compared to controls: 10 (2.9%) vs 22 (6.4%) (P = 0.03) (Figure 2b). Statistically significant difference was maintained in multivariable analysis with OR of 0.40, 95% CI: 0.16-0.96, P-value = 0.04 (Figure 3b). Factors significantly associated with increased odds of mechanical ventilation include age ≥65 (OR: 2.41, 95% CI: 1.04-5.60, P = 0.04), any pre-existing comorbid condition (OR: 3.45, 95% CI: 1.01-11.81, P = 0.048), baseline oxygen saturation <94% (OR: 5.86, 95% CI: 2.42-14.22, P <0.0001), neutrophil-to-lymphocyte ratio >3.3 (OR: 5.08, 95% CI: 2.11-12.25, P = 0.0003), hospital admission during dominance of pre-variants of concern strains vs Alpha (OR: 3.47, 95% CI: 1.20-10.04, P = 0.02) and hospital admission during dominance of Delta strain vs Alpha (OR: 3.65, 95% CI: 1.33-9.98, P = 0.01). Duration of symptoms was associated with reduced odds of new mechanical ventilation (OR: 0.82, 95% CI: 0.69-0.98, P = 0.03). (Figure 3b).
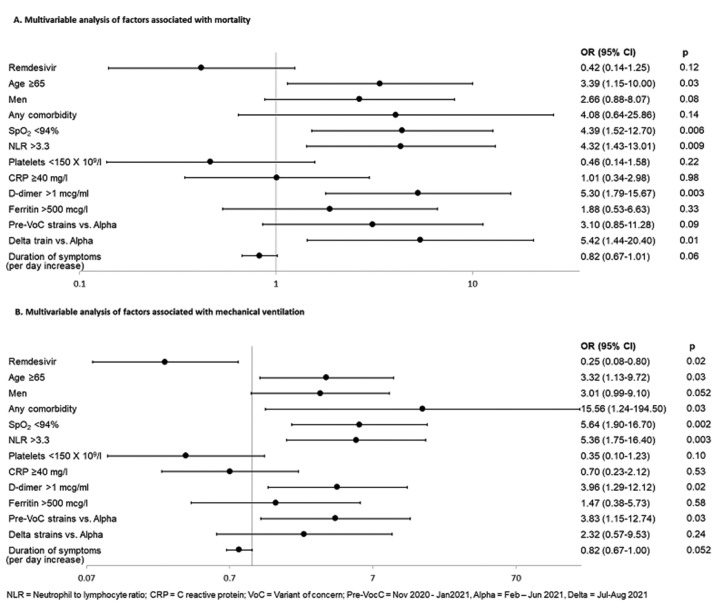
Similar results were obtained in a sub-group analysis of people receiving generic remdesivir (n = 265) and matched controls (n = 265). In crude analysis, remdesivir showed statistically significant improvement in mortality (2.3% in remdesivir and 5.7% in control groups, P = 0.045), as well as in the need for mechanical ventilation (2.0% in remdesivir and 6.4% in control groups, P = 0.01). In multivariable analysis, the benefit of remdesivir became insignificant (OR: 0.42, 95% CI: 0.14-1.25, P = 0.12) for mortality, while significance was maintained for the reduced odds of mechanical ventilation (OR: 0.25, 95% CI: 0.08-0.80, P = 0.02) (Figure 4 ).
Figure 4.
Multivariable analysis of factors associated with primary and secondary outcomes among persons receiving generic remdesivir (n = 530).
CI, confidence interval; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; SpO2, oxygen saturation on room air; VOC, variants of concern.
Discussion
We report results of comparative analysis of in-hospital mortality in Georgia's referral hospital for infectious diseases. Findings indicate a potential mortality benefit of remdesivir, which along with the reduction in the need for mechanical ventilation, strongly supports routine use of remdesivir for the treatment of hospitalized patients with COVID-19.
There has been uncertainty about the effectiveness of remdesivir in preventing death in the world literature. Results of clinical trials and observational studies range from no survival benefit through improved survival in certain patient populations to significant mortality reduction in all. THE WHO Solidarity trial showed no effect on mortality in any subset of patients, which led to WHO's guidelines panel recommending against the use of remdesivir [4,13]. Several studies, including pivotal ACTT-1, found survival benefits in a subset of patients, such as those requiring oxygen support without ventilation [3,14,15]. An observational study from France showed a reduction in the combined outcome of in-hospital death and/or intensive care unit admission [16]. Several other real-world studies, including a large observational study of more than 100,000 patients from the United States, showed significant survival benefit of remdesivir for all patients [5,[17], [18], [19], [20]]. Moreover, the results of a recent trial of outpatients showed that early initiation of remdesivir among high-risk non-hospitalized populations significantly reduces the risks of death [21].
Our data, showing a trend toward reducing mortality, does not refute but rather reinforces survival benefit of remdesivir. It should be noted that in our study, a similar proportion of patients received dexamethasone, and very few patients had been vaccinated at the time of hospital admission; therefore, we believe that the trend toward reducing mortality was due to remdesivir.
In addition to borderline effectiveness in terms of mortality, remdesivir, in our study, showed statistically significant improvement in the need for mechanical ventilation. This finding corroborates results of post hoc analysis of ACTT-1, a recent Canadian trial, and of two systematic reviews also showing benefits in preventing the new need for mechanical ventilation [14,[22], [23], [24]].
SARS-CoV-2 strains circulating in Georgia changed over time during the study period. This was accounted for in multivariable analysis by categorizing hospital admission periods into three categories based on dominant circulating strains in Georgia, according to GISAID. Approximately 30% of participants were admitted to hospital during the predominance of the Delta strain, which is known to cause more severe disease compared to previous variants, especially among unvaccinated persons [25]. This suggests that remdesivir can be beneficial for all variants of the virus.
To the best of our knowledge, our study is the first comparative effectiveness analysis of predominantly generic remdesivir from resource-limited settings. Most descriptive reports from India, Pakistan, and Nepal provided proof of concept that remdesivir can be safely and effectively rolled out in lower-income settings [26], [27], [28]. We further justify that remdesivir is a foundational treatment option for hospitalized patients with COVID-19 in resource-limited countries as well. Sub-group analysis of generic remdesivir showed similar results in terms of survival benefit in crude analysis and a statistically significant 75% reduction in the new need for mechanical ventilation in multivariable model. This latter finding is particularly important for low- and middle-income countries (LMIC). Even before the COVID-19 pandemic, invasive mechanical ventilation was associated with high rates of mortality in LMICs, reaching 72% compared to up to 34% in high-income countries [29]. This striking difference is likely due to the lack of technical and human capacities, which could have been even more critical in the environment of a stressed healthcare system during COVID-19 pandemic. Therefore, we strongly believe that averting the need for mechanical ventilation can bring substantial improvements in survival in LMICs.
Use of generics has important cost considerations for LMICs, especially considering the latest update from WHO of September 16, 2022, recommending remdesivir for severe COVID-19 [13]. Thanks to the Gilead's agreement with generic manufacturers, remdesivir is licensed for sale in 127 LMICs at a substantially lower cost compared to the brand product [30,31]. Economic analysis from South Africa showed that the use of generic remdesivir at a price of $330 for a 5-day treatment course could be cost-saving [32]. The price of the generic formulation has been reduced even more since the publication of this paper. Latest procurement prices from Georgia indicate that the 5-day treatment course may cost as low as $54, making it more affordable for even more countries.
Limitations
As with all non-randomized comparisons our study is subject to bias because of unmeasured or residual confounding. Propensity score matching was performed only on four variables (age, gender, comorbidities, and oxygen saturation) that are key factors in decision-making on the prescription of remdesivir in Georgia. This resulted in imbalance of other factors known to increase risk of mortality, such as inflammatory markers. This imbalance was accounted for in multivariable analysis. Analyses did not account for safety profiles and patients’ body mass index, the two factors that may have influenced the outcomes. However, it should be noted that obesity was identified as one of the comorbid conditions and was included in the analysis as part of the comorbid conditions. Also, analysis included deaths occurring during the hospitalization; we did not have information on vital status of patients after their discharge. With median of 9 days of hospital stay, it is possible that we missed fatal cases occurring after live discharge from our hospital. Finally, the relatively small sample size of the cohort affected statistical power. Despite these limitations, we strongly believe that the study meaningfully adds to the body of knowledge on the use of remdesivir in a real-world setting, especially in resource-limited settings.
In summary, this comparative effectiveness study showed clinically important benefits of remdesivir in terms of borderline reduction in the odds of death and statistically significant decrease in the need for mechanical ventilation. Although association with reduced mortality was not statistically significant, the direction of the effect suggests potential for clinically important survival benefits. Along with accumulating real-world evidence, our data support use of remdesivir in hospitalized patients with COVID-19 in both resource-rich and resource-limited countries, especially given that the drug is now recommended by WHO and is available as a low-cost generic formulation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Study was approved by the Institutional Review Board of Infectious Diseases, AIDS and Clinical Immunology Research Center, Tbilisi.
Author contributions
Concept and design (RM, TT, NC, AA), statistical analyses (RM, NC, AA), interpretation of the data (RM, TT, NC, AA, ME, ME, AB), drafting the manuscript (RM, TT, NC) and critical revision of the manuscript for intellectual content (RM, TT, NC, AA, ME, ME, AB). All authors read and approve the final manuscript.
Declaration of competing interest
The authors have no competing interests to declare.
References
- 1.Rubin D, Chan-Tack K, Farley J, Sherwat A. FDA approval of remdesivir - a step in the right direction. N Engl J Med. 2020;383:2598–2600. doi: 10.1056/NEJMp2032369. [DOI] [PubMed] [Google Scholar]
- 2.Kmietowicz Z. Covid-19: selected NHS patients will be treated with remdesivir. BMJ. 2020;369:m2097. doi: 10.1136/bmj.m2097. [DOI] [PubMed] [Google Scholar]
- 3.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Solidarity Trial Consortium. Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM, Hernández García C, Kieny MP, Malekzadeh R, Murthy S, Reddy KS, Roses Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E, Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P, Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L, Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L, Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T, Manevska S, Manuel O, McGinty S, Medina MT, Mesa Rubio ML, Miranda-Montoya MC, Nel J, Nunes EP, Perola M, Portolés A, Rasmin MR, Raza A, Rees H, Reges PPS, Rogers CA, Salami K, Salvadori MI, Sinani N, Sterne JAC, Stevanovikj M, Tacconelli E, Tikkinen KAO, Trelle S, Zaid H, Røttingen JA, Swaminathan S. Repurposed antiviral drugs for Covid-19 - interim WHO Solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mozaffari E, Chandak A, Zhang Z, Liang S, Thrun M, Gottlieb RL, et al. Remdesivir treatment in hospitalized patients with coronavirus disease 2019 (COVID-19): a comparative analysis of in-hospital all-cause mortality in a large multicenter observational cohort. Clin Infect Dis. 2022;75:e450–e458. doi: 10.1093/cid/ciab875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chokkalingam AP, Li H, Asubonteng J, Mozaffari E, Wang JR, Bush C, et al. Comparative effectiveness of remdesivir treatment in patients hospitalized with COVID-19. World Microbe Forum. 2021 [Google Scholar]
- 7.Go AS, Malenica I, Fusco D, Günthard HF, Ahn MY, Gupta SK, et al. Background Remdesivir vs Standard of care for severe COVID-19, 2021.
- 8.Garibaldi BT, Wang K, Robinson ML, Zeger SL, Bandeen-Roche K, Wang MC, et al. Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohl ME, Miller DR, Lund BC, Kobayashi T, Richardson Miell K, Beck BF, et al. Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalized with COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19), https://ourworldindata.org/coronavirus; 2022 [accessed 14 November 2022].
- 11.Tsertsvadze T, Ezugbaia M, Ratiani L, Abutidze A, Gokhelashvili A, Tchumburidze N, et al. Clinical management of COVID-19. Hopitalized Adult Patients_National Guideline. Tbilisi; Georgia: 2021. pp. 33–118. [Google Scholar]
- 12.The GISAID initiative. hCoV-19 variants dashboard, https://gisaid.org/hcov-19-variants-dashboard/; [accessed 28 October 2022].
- 13.World Health Organization . World Health Organization; Geneva: 2022. Guideline Therapeutics and COVID-19: living guideline. [PubMed] [Google Scholar]
- 14.Kaka AS, MacDonald R, Greer N, Vela K, Duan-Porter W, Obley A, et al. Major update: remdesivir for adults with Covid-19: a living systematic review and meta-analysis for the American College of Physicians practice points. Ann Intern Med. 2021;174:663–672. doi: 10.7326/M20-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garibaldi BT, Wang K, Robinson ML, Betz J, Caleb Alexander G, Andersen KM, et al. Real-world effectiveness of remdesivir in adults hospitalized with coronavirus disease 2019 (COVID-19): a retrospective, multicenter comparative effectiveness study. Clin Infect Dis. 2022;75:e516–e524. doi: 10.1093/cid/ciab1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gressens SB, Esnault V, de Castro N, Sellier P, Sene D, Chantelot L, et al. Remdesivir in combination with dexamethasone for patients hospitalized with COVID-19: a retrospective multicenter study. PLoS One. 2022;17 doi: 10.1371/journal.pone.0262564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benfield T, Bodilsen J, Brieghel C, Harboe ZB, Helleberg M, Holm C, et al. Improved survival among hospitalized patients with coronavirus disease 2019 (COVID-19) treated with remdesivir and dexamethasone. A nationwide population-based cohort study. Clin Infect Dis. 2021;73:2031–2036. doi: 10.1093/cid/ciab536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz GA, Christensen AB, Pusch T, Goulet D, Chang SC, Grunkemeier GL, et al. Remdesivir and mortality in patients with coronavirus disease 2019. Clin Infect Dis. 2022;74:1812–1820. doi: 10.1093/cid/ciab698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Vidal C, Alonso R, Camon AM, Cardozo C, Albiach L, Agüero D, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76:3296–3302. doi: 10.1093/jac/dkab321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olender SA, Walunas TL, Martinez E, Perez KK, Castagna A, Wang S, et al. Remdesivir versus standard-of-care for severe coronavirus disease 2019 infection: an analysis of 28-day mortality. Open Forum Infect Dis. 2021;8:ofab278. doi: 10.1093/ofid/ofab278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali K, Azher T, Baqi M, Binnie A, Borgia S, Carrier FM, et al. Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial. CMAJ. 2022;194:E242–E251. doi: 10.1503/cmaj.211698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ansems K, Grundeis F, Dahms K, Mikolajewska A, Thieme V, Piechotta V, et al. Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;8 doi: 10.1002/14651858.CD014962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paules CI, Gallagher SK, Rapaka RR, Davey RT, Doernberg SB, Grossberg R, et al. Remdesivir for the prevention of invasive mechanical ventilation or death in coronavirus disease 2019 (COVID-19): a post hoc analysis of the adaptive COVID-19 treatment Trial-1 cohort data. Clin Infect Dis. 2022;74:1260–1264. doi: 10.1093/cid/ciab695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ong SWX, Chiew CJ, Ang LW, Mak TM, Cui L, Toh MPHS, et al. Clinical and virological features of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.351 (Beta), and B.1.617.2 (Delta) Clin Infect Dis. 2022;75:e1128–e1136. doi: 10.1093/cid/ciab721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupte V, Hegde R, Sawant S, Kalathingal K, Jadhav S, Malabade R, et al. Safety and clinical outcomes of remdesivir in hospitalised COVID-19 patients: a retrospective analysis of active surveillance database. BMC Infect Dis. 2022;22:1. doi: 10.1186/s12879-021-07004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koirala J, Gyanwali P, Gerzoff RB, Bhattarai S, Nepal B, Manandhar R, et al. Experience of treating COVID-19 with remdesivir and convalescent plasma in a resource-limited setting: a prospective, observational study. Open Forum Infect Dis. 2021;8:ofab391. doi: 10.1093/ofid/ofab391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malik MI, Zafar SAF, Malik M, Qayyum F, Akram I, Arshad A, et al. The utility of remdesivir in SARS-CoV-2: a single tertiary care center experience from a developing country. Explor Res Clin Soc Pharm. 2022;5 doi: 10.1016/j.rcsop.2022.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inglis R, Ayebale E, Schultz MJ. Optimizing respiratory management in resource-limited settings. Curr Opin Crit Care. 2019;25:45–53. doi: 10.1097/MCC.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilead Sciences. Press releases: an open letter from Daniel O'Day, chairman & CEO, Gilead Sciences, https://www.gilead.com/news-and-press/press-room/press-releases/2020/6/an-open-letter-from-daniel-oday-chairman–ceo-gilead-sciences; 2020 [accessed 18 April 2022].
- 31.Mylan. Licensed countries for sale of remdesivir, https://www.desrem.in/en/row/supply-storage-and-handling/licensed-countries-for-sale-of-remdesivir; 2022 [accessed 19 April 2022].
- 32.Jo Y, Jamieson L, Edoka I, Long L, Silal S, Pulliam JRC, et al. Cost-effectiveness of remdesivir and dexamethasone for COVID-19 treatment in South Africa. Open Forum Infect Dis. 2021;8:ofab040. doi: 10.1093/ofid/ofab040. [DOI] [PMC free article] [PubMed] [Google Scholar]