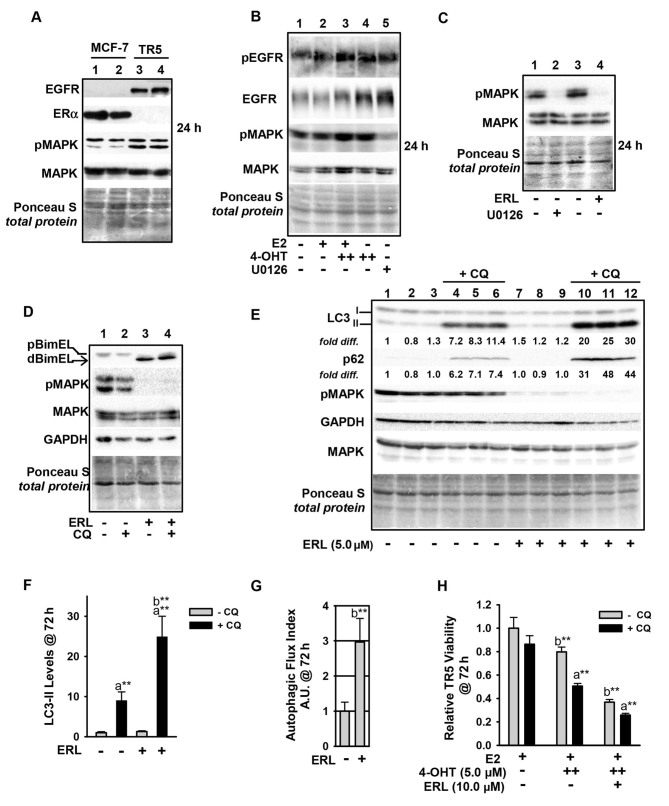
Figure 6.
Upregulation of the EGFR/MEK1/MAPK1/2 signaling axis in antiestrogen resistant TR5 cells. (A) TR5 cells express EGFR, concomitant with loss of ERα expression as shown by immunoblotting. (B) EGFR is phosphorylated at Tyr1068 in a MEK1/MAPK1/2 independent manner and under conditions of 4-OHT selection at 1 and 5 µM (designated as + and ++, respectively). (C and D) ERL, a selective tyrosine kinase inhibitor of EGFR utilized at 5 µM, blocks pMAPK1/2 phosphorylation at 24 h as effectively as U0126-mediated blockade of MEK1/MAPK1/2 (signal intensity of pMAPK compared in lanes 2 and 4) (C), and increases dBimEL levels in TR5 cells (D). (E) Immunoblot analyses (n=3) shows increased autophagy in 4-OHT-selected TR5 cells treated with 5 µM ERL for 72 h; LC3-II and p62 are elevated in treatments conducted in the presence of CQ indicating active flux in the ERL-treated cell populations. (F and G) A graphical representation of LC3-II steady-state levels, (F) and flux (G) are provided for LC3-II. Autophagic flux was calculated as described in materials and methods. (H) MTT assay shows a decrease in the relative viability of TR5 cells undergoing ERL treatment at 10 µM plus and minus CQ for 72 h. In panels A-E, signal intensity of total MAPK provides the loading control, was used to correct for loading variations to establish relative signal intensities (D and E), and to determine efficacy of U0126-mediated blockade of MEK (reduced pMAPK1/2 levels). Comparisons that show statistically significant differences includea CQ relative to the respective treatment conducted in the absence of CQ; bthe designated treatment compared with no treatment or growth in E2. **P<0.05. CQ, chloroquine; 4-OHT, 4-hydroxytamoxifen; p-, phosphorylated.