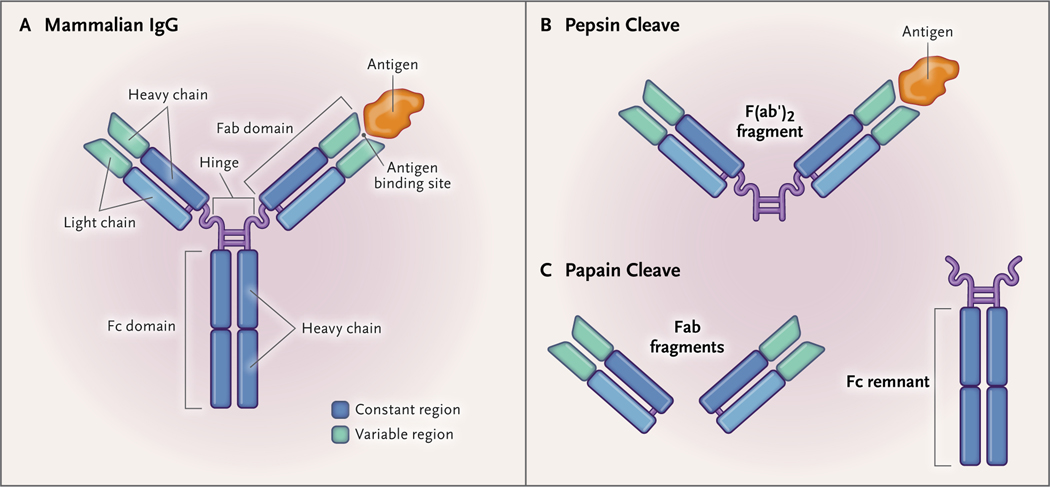
Figure 4. IgG and IgG Fragments Developed against Snake Venom Components.
The mammalian IgG molecule (Panel A) consists of an Fc (heavy) chain, a hinge, and two Fab (light) chains. The light chains have constant and variable regions, which allow the IgG to bind to certain antigens (Ag), such as venom components. When the IgG is treated with pepsin, the IgG molecule is cleaved below the hinge (comprising two disulfide bridges), and an F(ab′)2 fragment is produced (Panel B). When the IgG is treated with papain, the cleavage occurs above the hinge, and two Fab fragments are produced (Panel C). The Fc remnant or chain, which is more immunogenic than the Fab chains, can be removed from the remaining solution by means of various purification techniques.