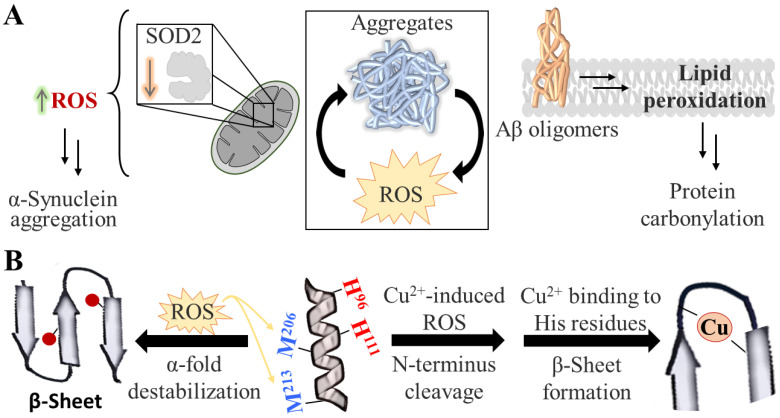
Figure 2.
Interplay between protein aggregates and oxidative damage. (A) Increased protein aggregation in several neurodegenerative diseases is associated with increased ROS levels. This relationship is bidirectional; excessive ROS production is found to induce protein aggregation and vice versa. Two examples of this vicious cycle are shown: (1) a decrease in the mitochondrial levels of Superoxide dismutase 2 (SOD2) gives rise to elevated ROS, which in turn promotes A-synuclein aggregation (right panel) and (2) the accumulation of Aβ oligomers induces lipid peroxidation, an oxidative damage that further results in protein carbonylation (left panel). (B) Proposed mechanisms of ROS-induced aggregation of prion protein. At physiological conditions, part of the prion protein exists in the α-fold form (depicted as a helix in the middle). However, in disease, its scrapie isoform forms β-sheets, which eventually drive fibrillation. The transition from α-fold to β-sheet is proposed to be mediated by ROS, with two different mechanisms: (1) by methionine oxidation (depicted as red circles), which destabilizes the α-fold form (left side) or (2) by ROS-mediated cleavage—induced by copper—of the protective N-terminus of prion, and subsequently, β-sheet formation due to copper chelation by neighboring histidine residues. The position of the residues shown is for illustration only and do not correspond to their true location.