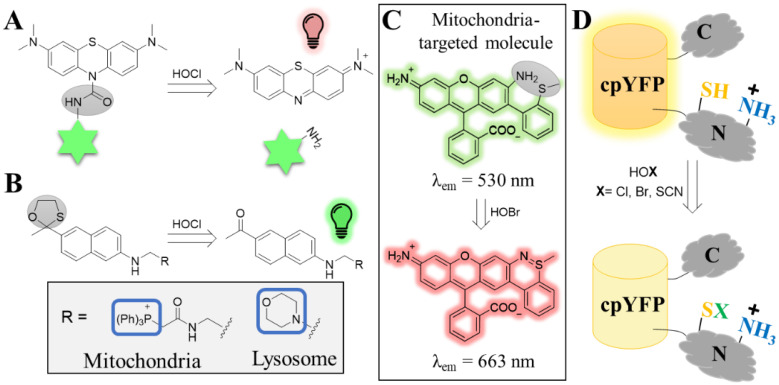
Figure 3.
Tools for the visualization of hypohalous acids. (A) Structure and turn-on mechanism of the methylene blue-based probe (FDOCl-18) upon sensing HOCl. The methylene blue (top structure) is conjugated to naphthalene (indicated as a green star) through the HOCl-sensing group (highlighted by the gray oval, left structure). The methylene blue is non-fluorescent while the sensing group is attached. Upon sensing HOCl, a deformylation mechanism takes place that leads to the liberation of the methylene blue, which is now fluorescent. (B) Sensing mechanism of the organelle-specific turn-on probe for HOCl. The fluorescence properties of a two-photon dye (acedan) were quenched by HOCl-labile group (gray circle, left structure). The probe was further equipped with organelle specific-targeted moieties to drive it either to mitochondria (MITO-TP) or to lysosomes (LYSO-TP). (C) Structure of the HOBr ratiometric probe RhSN-mito. In the absence of HOBr, the probe emits at 530 nm. Reaction of the sensing moiety (gray oval, top structure) with HOBr leads to the formation of an additional ring that alters the fluorescence properties of the probe, which now emits at 663 nm. (D) The NemR-cpYFP construct (Hypocrates) for detecting hypohalous acids. The YFP was integrated onto a flexible loop of the NemR. While the YFP exhibits the high fluorescence in non-oxidation conditions, the intensity decreases when the thiol of the cysteine senses the hypohalous acid.