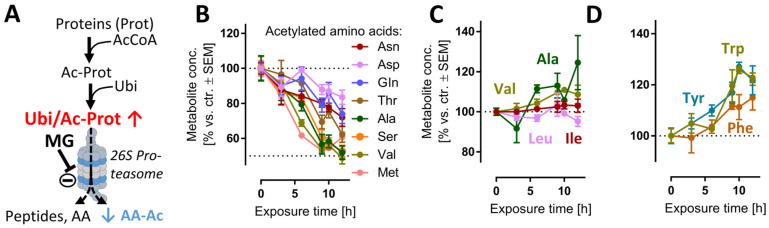
Figure 2.
Reduced amounts of acetylated amino acids as evidence for proteasome inhibition. Cells were treated as described in Figure 1 to obtain time-dependent metabolomics data, following addition of 100 nM MG. Data on acetylated amino acids (AA-Ac) and some other amino acids (AAs) were extracted from the metabolome data matrix. (A) Chain of events leading to the generation of AA-Ac. Post-translational modification of proteins by the acetyl group leads to polypeptide chains containing AA-Ac. The ubiquitination (Ubi) of these proteins (Ubi/Ac-Prot) can lead to their degradation by the 26S proteasome. This process yields: amino acids, small peptides and AA-Ac. (B) Time course of the levels of AA-Ac over time. (C,D) Quantification of hydrophobic and aromatic AAs over time. Data are means ± SEM of independent replicates. For MG treatments, 3 different samples were analyzed. For controls (DMSO), 4 replicates were prepared to provide for more robust baseline data. AcCoA, acetyl coenzyme A; MG, proteasome inhibitor MG-132.