Table 1.
Conventionally approved PS employed in PDT for cancer treatment.
| Photosensitizer | Generic Name | λ (nm) Max. |
Chemical Structure |
Drug Light Interval | Approved for | Ref. |
|---|---|---|---|---|---|---|
| 5-Aminolevulinic acid (ALA) | Luvalan | 635 |
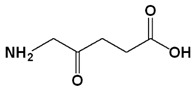
|
6 h | Actinic keratosis (USA 1999) | [32,33] |
| Hematoporphyrin derivatives (HPD) | Photofrin (Porfmer sodium) | 630 |
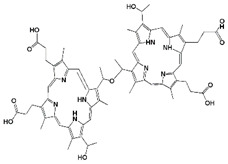
|
48 h | (i) Bladder cancer (Canada 1993) (ii) Early-stage lung cancer (Japan 1994) (iii) Esophageal cancer (FDA USA 1995), Early-stage non-small-cell lung cancer (FDA USA 1998) |
[29,31] |
| Meta-tetra(hydroxyphenyl) chlorin (mTHPC) | Foscan (temoporfin) | 652 |
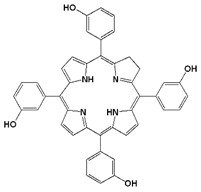
|
96 h | (i) Head and neck squamous cell carcinoma (Europe 2001) | [29,31] |
| Benzoporphyrin derivative monoacid ring A | Verteporfin or Visudyne | 690 |
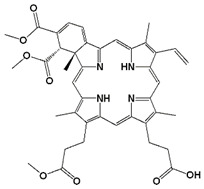
|
30 min | Choroidal neovascularization (age-related macular degeneration (AMD) (FDA 2000)) | [34] |
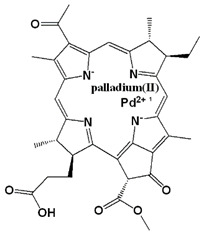
| Palladium (Pd)—substituted bacteriochlorophyll derivative | Tookad (WST09 (padoporfin) WST11 (padeliporfin or TOOKAD Soluble)) |
763 |
|
Short interval mins | Clinical trial for prostate cancer | [29,35,36] |
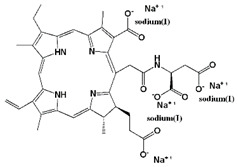
| N-aspartyl chlorine e6 (NPe6) | Talaporfin sodium (Laserphyrin®) | 664 |
|
0.25–4 h | (i) Early-stage lung cancer (Japan 2003) | [12,29] |
