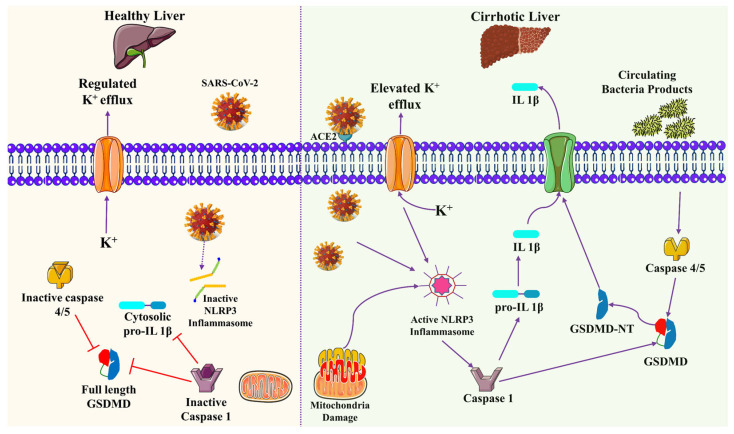
Figure 5.
Cirrhosis-related upregulation of hepatocyte inflammasome signaling predisposes increased cell death after SARS-CoV-2 infection. Bacterial substances (such as lipopolysaccharide) bind to and activate caspases-4/5 in cirrhosis (right panel), causing the dimeric protein Gasdermin-D to be cleaved (GSDMD). The N-terminus of GSDMD migrates to the plasma membrane, where it generates holes that allow damage-associated molecular patterns and electrolytes to flow uncontrollably. NLRP3 assembly is also triggered by K+ efflux and mitochondrial injury, which activates caspase-1 and results in pro-IL1 processing. Virus proteins attach to the previously synthesized NLRP3, initiating downstream pathways during SARS-Cov-2 infection. In contrast, the absence of the ACE2 receptor in healthy livers (left panel) delays SARS-CoV-2 entry into cells. NLRP3 is present but inactive, preventing proinflammatory caspase activation and processing GSDMD and pro-IL1. This figure is reproduced from Luo et al. [65] (Attribution 4.0 International (CC BY 4.0)).