Figure 3. Kaplan-Meier Plots for Progression-Free Survival and Duration of Response in Low Programmed Death Ligand 1 (PD-L1) Subgroups Derived With KMSubtraction.
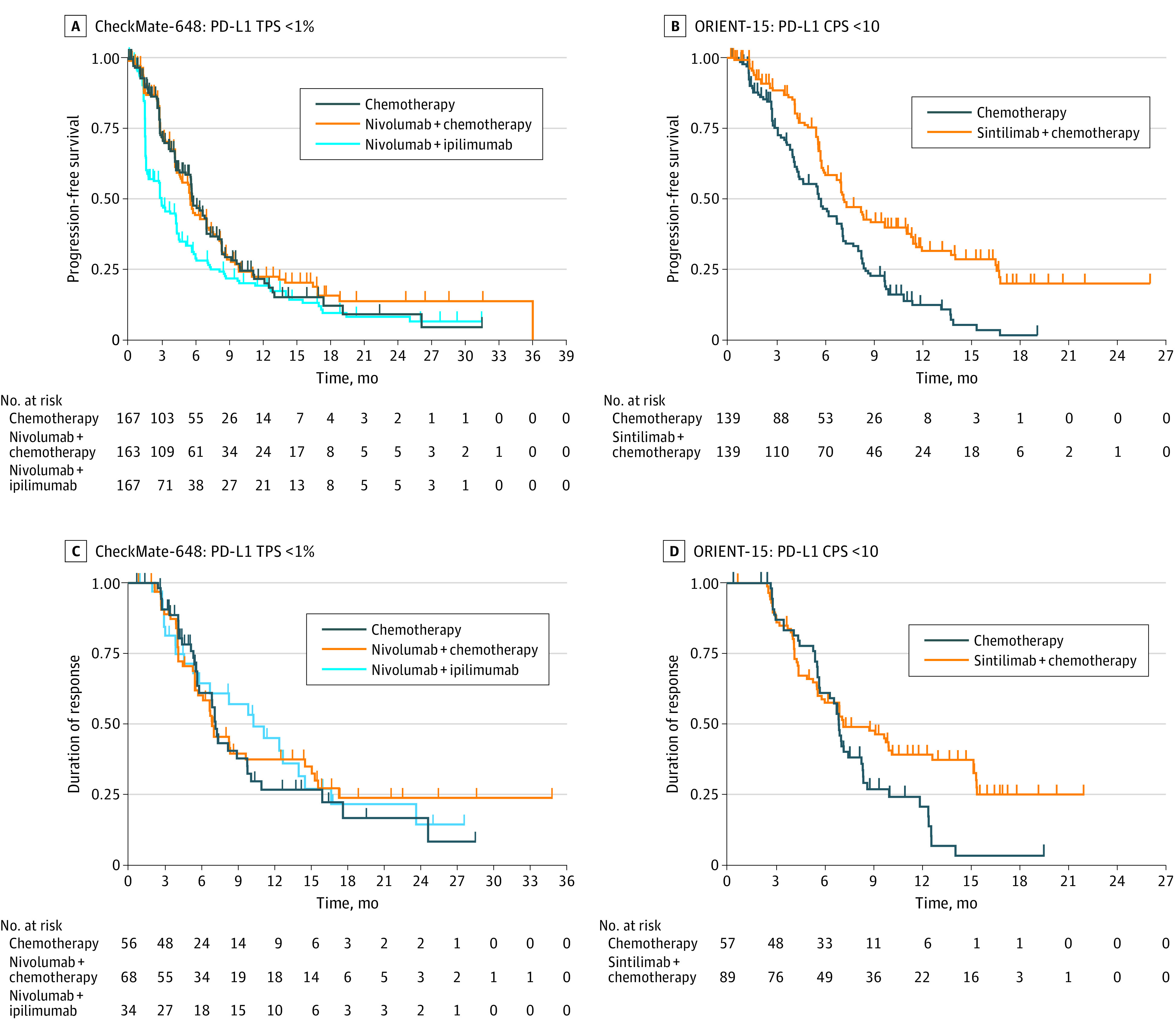
A, In the subgroup of CheckMate-648 with a tumor proportion score (TPS) lower than 1%, no significant difference in progression-free survival was observed between immunochemotherapy and chemotherapy (hazard ratio [HR], 0.98; 95% CI, 0.75-1.28; P = .88); there was a significantly inferior progression-free survival for ipilimumab plus nivolumab dual immunotherapy compared with chemotherapy alone (HR, 1.47; 95% CI, 1.14-1.90; P = .003). B, In the subgroup of ORIENT-15 with a combined positive score (CPS) lower than 10, a significant difference in progression-free survival was observed between immunochemotherapy and chemotherapy (HR, 0.52; 95% CI, 0.39-0.70; P < .001). C, In the subgroup of CheckMate-648 with a TPS lower than 1%, the median duration of response was 6.9 months (95% CI, 5.8-15.1 months; HR, 0.91; 95% CI, 0.58-1.44; P = .70) for nivolumab-based immunochemotherapy, 10.3 months (95% CI, 5.8-16.7 months; HR, 0.87; 95% CI, 0.51-1.49; P = .61) for nivolumab plus ipilimumab, and 7.2 months (95% CI, 5.8-10.1 months) for chemotherapy. D, In the subgroup of ORIENT-15 with a CPS lower than 10, the median duration of response was 7.2 months (95% CI, 5.8-15.2 months; HR, 0.64; 95% CI, 0.43-0.96; P = .03) for sintilimab-based immunochemotherapy and 6.9 months (95% CI, 5.7-8.4 months) for chemotherapy.
